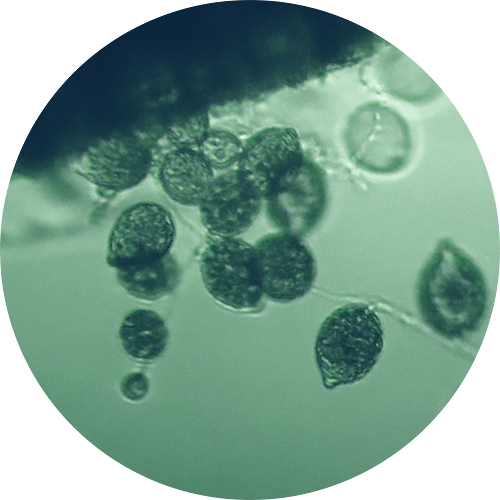
A large number of studies over several decades have shown that Phytophthora diseases are common in many plant nurseries, particularly those growing ornamental plants (Yakaba et al 2009, Beinapfl and Balci 2014, Parke et al 2014, Jung et al 2015). Previous reports (Swiecki et al 2011) and recent data (Reeser et al 2015, Rooney-Latham et al 2015a,b, Rooney-Latham et al 2014) show that Phytophthora diseases are similarly common in nurseries producing native plants. Nursery stock has become the most common means for the introduction of new Phytophthora species into landscapes and habitats worldwide (Brasier 2008, Liebhold et al 2012).
To understand why Phytophthora species are so common in nurseries, we need to understand how plant diseases develop and spread, the disease cycle of Phytophthora root rots, and how nursery conditions relate to these.
The development of plant diseases caused by biotic pathogens such as Phytophthora is commonly described through a simple conceptual model known as the plant disease triangle. This simplified model indicates that disease development requires a favorable alignment of three factors: host plant, pathogen, and environment. A key concept conveyed by the plant disease triangle is that removing any part of the triangle – susceptible host plant, virulent pathogen, or favorable environment – will prevent disease from developing.
Adding two more elements to this model provides a more complete picture of the factors that interact in plant disease development (Figure 1). For many diseases, especially those caused by soil-borne pathogens, the activity of other microorganisms may favor or inhibit the activity of the pathogen. One way to add this to the model is to divide the environment factor into two components: biotic (other interacting organisms) and abiotic (physical factors). The biotic environment interacts with both the pathogen and the host and is influenced by abiotic environmental factors such as temperature and moisture. Finally, the interactions between these four sets of factors all occur through time. Infection and pathogen growth, reproduction, and dispersal are processes that require time, and disease development can be inhibited if the convergence of factors that favor disease do not persist for a sufficient amount of time. Variations of this model are known as the plant disease pyramid.
While host susceptibility or resistance to a pathogen strongly affect disease severity on individual plants, other host-related factors influence disease spread through a population of plants. Pathogen spread from plant to plant and the build-up of pathogen populations are strongly related to the density of susceptible hosts and the availability and abundance of susceptible host tissues. Rapid buildup of pathogen inoculum and a high rate of infection are more likely to occur where susceptible hosts are found at high densities.
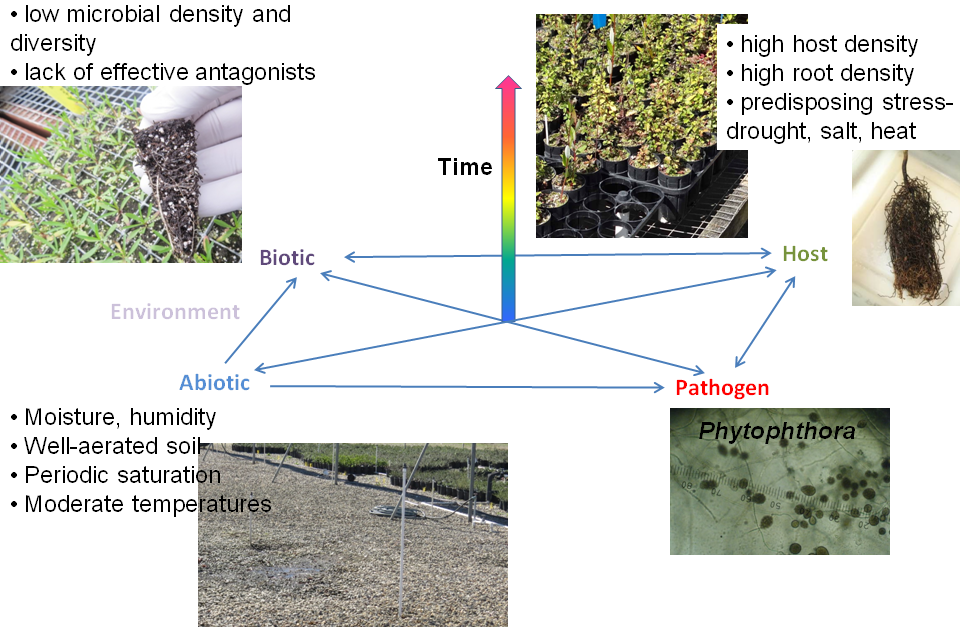
Figure 1. Diagram of the plant disease pyramid, showing interactions between various factors that affect the development of plant diseases in nurseries. Disease will not develop if any of the factors at the base of the pyramid absolutely limit disease (e.g., resistant host, lack of pathogen, completely antagonistic microbial environment, unsuitable temperature and moisture) or if favorable conditions do not persist long enough. Reciprocal interactions exist between most factors (blue arrows). For example, host plants are affected by the abiotic environment (e.g., high temperature, low soil moisture can induce water stress) and the local abiotic environment can be affected by host plant (e.g., plant canopy can reduce soil temperature, increase humidity). The main exception is that pathogenic and nonpathogenic microorganisms generally have little direct effect on the abiotic environment.
Phytophthora is a member of the Oomycetes or water molds, and the life cycle of Phytophthora is strongly influenced by the presence of water. Much of this is associated with fact that Phytophthora produce motile spores (zoospores) that use flagella to actively swim in water. Zoospores are attracted by various chemicals, including substances that diffuse from host tissues. This allows them to actively seek out host tissues, such as roots in the soil, and to aggregate on the host surface, where they can initiate massed attacks. However, massed attacks are not required for zoospore infection of susceptible host tissue. Even small numbers of zoospores can initiate an infection of a susceptible host.
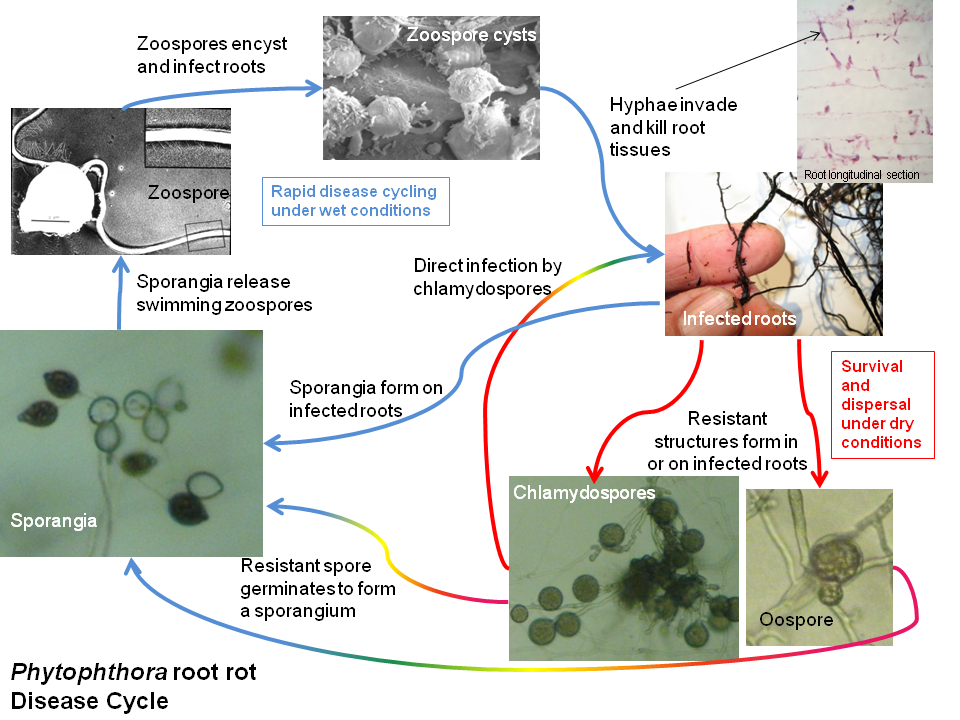
A generalized disease cycle for Phytophthora root rots is shown in Figure 2. Rapid disease cycling (Figure 2, upper half) occurs under moist conditions that include at least brief periods of soil saturation. Under favorable conditions, sporangia are produced on the surface of infected roots or from resistant spores such as chlamydospores and oospores. Sporangium production is favored by moist, well-aerated conditions but does not require soil saturation. When sporangia are exposed to free water, such as water-filled pores in soils wetted by rain or irrigation, each can release many (about 20 to 50) zoospores, which can swim for many hours. Zoospores actively swim upwards (which facilitates dispersal via water splash) and toward host roots. Upon reaching a root, the zoospore adheres to it and forms a spherical cyst. Within a few hours, the cyst germinates, producing hyphae that penetrate the host root and begin growing through and between host cells. Within 24 hours from infection, sporangia can be formed on newly infected host roots.
Phytophthora kills the host cells it invades as well as adjacent cells. Within a day or less, secondary organisms can also invade and begin to degrade dead and dying root tissues. It can be difficult to detect or isolate Phytophthora in infected roots using available diagnostic techniques once they become colonized and degraded by these secondary organisms. Phytophthora hyphae can continue to grow through roots, including the vascular tissues, until either all suitable host tissues are killed or the host is able to limit pathogen expansion through its defense reactions.
Phytophthora hyphae can produce resistant survival structures on or in invaded host tissues (Figure 2, lower right). Depending on the species, these may include spherical asexual spores (chlamydospores) or sexual spores (oospores), or thick-walled hyphae. These structures can tolerate drying and persist in dead root fragments or in the soil. In the presence of appropriate stimuli, including moist conditions and root exudates, these resistant structures give rise to hyphae that can either directly infect roots or produce sporangia.
A few key points:
Host: Nursery grown plants feature high root density within containers and close spacing of plants, providing near optimum conditions for disease spread and reproduction. Plant handling and frequent rearranging of containers further increase opportunities for pathogen spread. Because of the limited soil volume in containers, plants may be water stressed periodically if irrigation does not keep up with water use. Episodes of water stress or salt stress (also common in container grown plants) can make plants more susceptible to Phytophthora infection.
Abiotic environment: Nursery plants are normally maintained under moist and somewhat humid conditions. This provides ample moisture for sporangium production along with intermittent flooding to promote zoospore release and dispersal. Potting media are normally well-aerated, which favors sporangium production, and have large pore spaces, which facilitate zoospore movement. Even if the potting medium drains quickly, the bottom of a container normally remains saturated for an extended period. Temperatures are also maintained in a moderate range suitable for many Phytophthora species.
Biotic environment: Nursery containers are simplified systems that normally lack the density and diversity of microorganisms that are found in soils that suppress Phytophthora root diseases. It is difficult to artificially introduce and maintain microbial antagonists at levels needed to strongly suppress Phytophthora in any system, including nursery containers.
Time: Conditions favorable for infection and disease development commonly persist as long as plants are maintained in the nursery. Plants that are kept in the nursery longer have the more chances of being contaminated and more time for reinfection and disease development.
The bottom line is that host and environmental factors in the nursery cannot be altered enough to prevent Phytophthora root diseases. Exclusion of the pathogen provides the only viable option for producing nursery plants that are free of Phytophthora. If the pathogen is not present, disease will not develop even if host and environmental conditions favor disease development.
Unfortunately, few nurseries have made the efforts needed to exclude Phytophthora. In fact, production practices common in many nurseries have provided many opportunities for contaminating nursery stock with Phytophthora. These practices include:
Best Management Practices (BMPS) for Producing Clean Nursery Stock details methods for producing nursery plants that are free of Phytophthora. Baker (1957) summed up the situation of managing pathogens in the nursery quite succinctly: “Don’t fight ‘em, eliminate ‘em”. The artificial situation that exists within container nurseries was designed to grow as many plants in as small a space as possible, as quickly as possible. As an unintended consequence, nurseries are also highly favorable for the development and spread of various plant diseases, including Phytophthora root diseases.
In essence, nursery grown plants are a human-developed convenience. All plants in native communities have evolved to establish and grow without first being established in containers. Planting diseased but “convenient” nursery stock can permanently infest both cultivated landscapes and native habitats with exotic pathogens. Introduced pathogens such as Phytophthora species can adversely affect the long-term health, management, and sustainability of these landscapes and habitats. This is an unintended consequence that can be avoided by using planting materials that are free of these pathogens.
Baker, K.F. (Editor). 1957. The U.C. System for Producing Healthy Container Grown Plants, Manual 23. University of California, Division of Agricultural Sciences, Agricultural Experiment Station Extension Service
Bienapfl, J. C., and Balci, Y. 2014. Movement of Phytophthora spp. in Maryland’s nursery trade. Plant Dis. 98:134-144.
Brasier, C.M. 2008. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathology 57(5):792-808.
Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; Corcobado, T.; Cravador, A.; Decourcelle, T.; Denton, G.; Diamandis, S.; Dogmus-Lehtijärvi, H.T.; Franceschini, A.; Ginetti, B.; Glavendekic, M.; Hantula, J.; Hartmann, G.; Herrero, M.; Ivic, D.; Horta Jung, M.; Lilja, A.; Keca, N.; Kramarets, V.; Lyubenova, A.; Machado, H.; Magnano di San Lio, G.; Mansilla Vázquez, P.J.; Marçais, B.; Matsiakh, I.; Milenkovic, I.; Moricca, S.; Nagy, Z.Á.; Nechwatal, J.; Olsson, C.; Oszako, T.; Pane, A.; Paplomatas, E.J.; Pintos Varela, C.; Prospero, S.; Rial Martínez, C.; Rigling, D.; Robin, C.; Rytkönen, A.; Sánchez, M.E.; Scanu, B.; Schlenzig, A.; Schumacher, J.; Slavov, S.; Solla, A.; Sousa, E.; Stenlid, J.; Talgř, V.; Tomic, Z.; Tsopelas, P.; Vannini, A.; Vettraino, A.M.; Wenneker, M.; Woodward, S.; and Peréz-Sierra, A. 2015. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. Forest Pathology. DOI: 10.1111/efp.12239.
Liebhold, A. M., Brockerhoff, E. G., Garrett, L. J., Parke, J. L., and Britton, K. O. 2012. Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Frontiers in Ecology and the Environment 10: 135–143.
Migliorini, D., Ghelardini, L., Tondini, E., Luchi, N., Santini, A. 2015. The potential of symptomless potted plants for carrying invasive soilborne plant pathogens. Diversity and Distributions 21: 1218–1229. doi: 10.1111/ddi.12347
Parke, J.L.; Knaus, B.J.; Fieland, V.J.; Lewis, C; Grünwald, N.J. 2014. Phytophthora community structure analyses in Oregon nurseries inform systems approaches to disease management. Phytopathology 104(10):1052-62.
Reeser, P. W.; Sutton, W.; Hansen, E. M.; Goheen, E. M.; Fieland, V. J.; Grunwald, N. J. 2015. First report of Phytophthora occultans causing root and collar rot on Ceanothus, boxwood, rhododendron, and other hosts in horticultural nurseries in Oregon, USA. Plant Disease 99:1282.
Rooney-Latham, S.; Blomquist, C.L. 2014. First Report of Root and Stem Rot Caused by Phytophthora tentaculata on Mimulus aurantiacus in North America. Plant Disease. 98(7): 996.
Rooney-Latham, S.; Blomquist, C.; Swiecki, T.; Bernhardt E. 2015a. Phytophthora tentaculata. Forest Phytophthoras 5(1). DOI: 10.5399/osu/fp.5.1.3727.
Rooney-Latham, S.; Blomquist, C.; Swiecki, T.; Bernhardt, E.; Frankel, S.J. 2015b. First detection in the USA: New plant pathogen, Phytophthora tentaculata, in native plant nurseries and restoration sites in California. Native Plants Journal 16:23-25.
Rooney-Latham, S.; Blomquist, C.; Swiecki, T.; Bernhardt, E.; Natesan, E.; Frankel, S.J.; 2014, November. Phytophthora detections in native plant nurseries and restoration sites in California. Paper presented at Phytophthora in Forests and Natural Ecosystems, Esquel, Argentina. Abstract retrieved from https://www.researchgate.net/profile/S_Rooney-Latham/publications.
Yakabe, L. E., Blomquist, C. L., Thomas, S. L., and MacDonald, J. D. 2009. Identification and frequency of Phytophthora species associated with foliar diseases in California ornamental nurseries. Plant Dis. 93:883-890.