Plant nurseries represent nearly optimal environments for the development and spread of Phytophthora diseases (see Plant Nursery Conditions Favor Phytophthora Root Rots). Hence, it is not surprising that many nurseries routinely use systemic chemicals that suppress Phytophthora diseases. These chemicals are commonly known as “fungicides”, a term that doesn’t really apply here. Phytophthora is a member of the water molds (Oomycetes), a group of microorganisms not related to true fungi. Furthermore, the systemic chemicals in question do not actually kill Phytophthora at normal use rates. For the purposes of this document, we will describe these materials as systemic oomycete suppressive (SOS) chemicals rather than use the inaccurate term “fungicides”. Although SOS chemicals can decrease economic losses caused by Phytophthora in nurseries, their use has not stopped the proliferation of Phytophthora in these environments. Despite the common use of SOS chemicals in nurseries, many studies have shown that a wide variety of Phytophthora species are present in nursery stock in (e.g., Yakabe et al 2009, Beinapfl and Balci 2014, Parke et al 2014, Jung et al 2015).
To unravel this apparent paradox, it is necessary to understand how these chemicals work. Two classes of systemic chemicals with strong inhibitory activity against water molds have become widely used since they were introduced in the late 1970’s.
Phenylamides, including metalaxyl (Subdue®) and mefenoxam (Subdue Maxx®, actually one of the two optical isomers present in metalaxyl) are systemic materials. They primarily move upward in plants, but can move downward to a limited degree. They have primarily protectant activity. Phenylamides do not affect zoospore release, germination, or penetration, but rapidly inhibit further development of the pathogen in infected hosts. They inhibit ribosomal RNA synthesis by specific interference with activity of a nuclear RNA-polymerase - template complex. This single-site activity poses a risk for the development of fungicide resistance, which develops most readily when these materials are used repeatedly against foliar-infecting Phytophthora species (Hill and Hausbeck 2008). Residual activity in treated plants can last for 70-90 days, or longer under some conditions.
Phosphonates include potassium and other salts of phosphorous acid (e.g., Agri-Fos®, Reliant®) as well as fosetyl aluminum (Aliette®). For all of these materials, the active compound is phosphite ion (PO3-3). Phosphonate (= phosphite ) is highly systemic. It moves both upward and downward in plants, but tends to accumulate at phosphorus sinks within plants, including developing fruits and roots. Plants do not utilize absorbed phosphonate as a source of phosphorus, though soil microorganisms can slowly convert it to phosphate (PO4-3), the form of phosphorus used by plants as a nutrient. Phosphate has no activity against oomycetes, whereas phosphonate has protectant activity against Phytophthora and other some other oomycetes. Zoospore production in infected plants is reduced, but not completely prevented by phosphonate.
The mode of action of phosphonate is complex. Phosphonate shows direct toxicity to Phytophthora species at high concentrations. At lower concentrations, phosphonate acts indirectly, by increasing a plant's natural resistance response to Phytophthora infection, inhibiting pathogen development in infected tissue. Disease suppression by phosphonate varies among plant species and even within varieties of a species. Residual activity can be as long as two years or more in field-grown plants.
A number of other SOS chemicals are registered for use against Phytophthora diseases. Depending on the chemical group, these chemicals differ with respect to many properties, including:
Nonetheless, it is critical to understand that all of these SOS chemicals suppress but do not eliminate Phytophthora infections. At normal use rates, they do not kill Phytophthora species in infected plant tissues. By way of illustration, Figure 1 shows the degree to which colony growth of a P. cinnamomi isolate is suppressed by metalaxyl. While the growth of the pathogen is substantially suppressed at relatively low doses that are within typical residue tolerance range (mostly 1 ug/ml or less), some growth still occurs at concentrations 10 times this level. Note that scientific papers studying SOS effects on Phytophthora may include chemical rates in excess of label rates.
If a plant is treated with an SOS chemical, the plant can still become infected by Phytophthora (Benson 1987, Hamm et al 1984, Tjosvold et al 2008). If levels of these chemicals are high enough in the host tissue, subsequent growth and reproduction of the pathogen will be suppressed. This can reduce disease cycling and symptom development, but does not kill the pathogen in the infected host tissues.
A variety of other factors come into play that can further affect the efficacy of chemical control measures. Although SOS chemicals are systemic, concentrations in treated plants will vary between tissues because of differences in tissue age, uptake and translocation, and other factors. Because of varying chemical levels within plants, pathogen development may be suppressed in some roots but not others. Infected plants treated with SOS chemicals remain infected and disease cycling will still occur, even if it is slowed. A similar scenario plays out at the larger scale of a treated nursery block or field. Chemical applications are not completely uniform in most situations. Some entire plants may have doses that are too low to be effective. In a population of plants treated with SOS chemicals, disease can still develop and spread.
Although Phytophthora is not completely inhibited in plants treated with SOS chemicals, disease can be reduced to the point that symptoms are suppressed, mortality is reduced or eliminated, and the appearance and yields of infected plants may be similar to those of uninfected plants. Combined with other disease management tactics, disease suppression via use of SOS chemicals can be a viable management strategy in the field where Phytophthora species have been introduced and eradication is not feasible.
However, the benefits associated using SOS chemicals on infected plants will not continue if chemical applications are discontinued. As chemical concentrations in plants drop over time, fewer roots will have concentrations high enough to suppress disease, and disease cycling will increase. Suppressed infections may also reactivate once SOS chemical residues decline. After a period of time, if environmental conditions are favorable, disease levels will increase and symptoms will become evident.
When SOS chemicals are used in nurseries, they can be effective in suppressing symptom development in infected plants. However, if nurseries follow cultural practices that continue to allow Phytophthora introduction and spread of these pathogens, using SOS chemicals will result in nursery stock that is infected but shows few or no symptoms of disease. From the standpoint of the nursery, these chemicals are reducing plant losses and increasing the number of saleable plants.
But what happens when these plants are purchased and planted out? If the consumer buys symptomless infected plants, they have a product with a concealed defect. After planting, levels of SOS chemicals in the plants will decline and Phytophthora activity can increase. Affected plants may simply grow slowly, or may eventually decline and die. Obvious symptom development may be delayed for months to years after planting, especially in woody plants. With this delay, few people make the connection between the dead or dying plant and the original infected nursery stock.
While this outcome alone is undesirable, the situation is made worse by the fact that Phytophthora-infected plants can also infest the planting site. Even if the affected plant is removed, the pathogen can remain behind in dead root fragments and resistant spores that can infect the next planting. Worse yet, replanting may introduce additional Phytophthora species to the planting site. Because Phytophthora host ranges vary widely, the effective host range of a mixed Phytophthora infestation is usually wider than that of any single species. If a site has a mixed Phytophthora infestation, the list of species that may tolerate or resist infection will become shorter and future planting options may be greatly restricted. Roots of adjacent susceptible host plants that extend into the infested soil can also be infected, increasing the size of the infested area over time. Finally, infested areas can serve a source of pathogen spread to other areas, where either planted or native vegetation may be infected.
As noted above, use of SOS chemicals may mask infected plants by reducing symptom development and expression. To overcome this, a common recommendation is to hold plants for a period after receiving them (6 to 8 weeks is commonly recommended) and then inspect or test the plants at that point. While this may help, the residual activity of these chemicals can be much longer than 8 weeks, especially if plants have been treated at high rates in the nursery. Furthermore, disease activity in the plants will not resume suddenly after a specific date. Generally, disease activity will increase gradually over many weeks or months as the concentration of SOS chemical drops and increasing numbers of roots become infected. Rates of disease progress and symptom expression will also vary with temperature, water regime, and other environmental factors. Finally, many drought-tolerant California native plants can sustain very high levels of Phytophthora root rot without showing obvious shoot symptoms. To increase chances of detecting Phytophthora in plants that may have been treated with SOS chemicals, a holding period of at least 3 to 4 months is recommended, followed by a sensitive testing method such as baiting.
What’s the bottom line for purchasers of nursery stock and nurseries that want to produce Phytophthora-free stock?
1. If a nursery has Phytophthora-infected plants, using SOS chemicals will not “clean up” the stock. Infected stock + SOS chemical = infected stock.
2. If a nursery is following a systems approach to clean plant production, SOS chemicals are not necessary. No pathogen = no disease.
3. Using chemicals to suppress Phytophthora in nurseries can thwart the quality-control testing that is needed to quickly identify and eradicate any Phytophthora infestation that may arise though lapses in a clean production system.
In effect, SOS chemicals have allowed nurseries to ignore clean production practices that exclude Phytophthora and adopt suppression as their main disease management strategy. As a result, the use of SOS chemicals by nurseries may actually increase the likelihood that the stock is infected by Phytophthora . A full systems approach to producing clean nursery stock provides the best route for producing Phytophthora -free plants (see Best Management Practices (BMPs) for Producing Clean Nursery Stock). Nurseries following this approach will not need to use SOS chemicals to “save our stuff”.
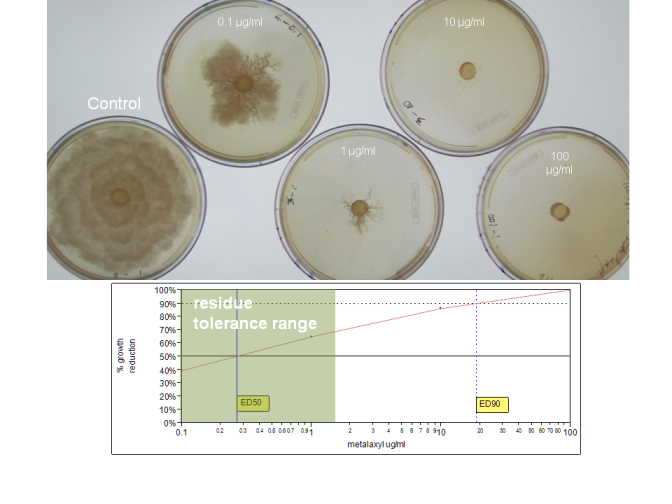 Figure 1. Growth suppression of a P. cinnamomi isolate by varying concentrations of metalaxyl. ED50 and ED90 values plotted in graph indicate concentrations that suppress colony growth by 50% and 90%, repectively. Most established residues for metalaxyl on food crops are in the shaded range shown in the graph. This isolate is sensitive to metalaxyl. Metalaxyl-resistant strains can grow with minimal suppression at metalaxyl concentrations of 100 µg/ml or more. Metalaxyl treatment will not suppress disease in a plant infected with a metalaxyl-resistant strain.
Figure 1. Growth suppression of a P. cinnamomi isolate by varying concentrations of metalaxyl. ED50 and ED90 values plotted in graph indicate concentrations that suppress colony growth by 50% and 90%, repectively. Most established residues for metalaxyl on food crops are in the shaded range shown in the graph. This isolate is sensitive to metalaxyl. Metalaxyl-resistant strains can grow with minimal suppression at metalaxyl concentrations of 100 µg/ml or more. Metalaxyl treatment will not suppress disease in a plant infected with a metalaxyl-resistant strain.
Benson, D. M. 1987. Occurrence of Phytophthora cinnamomi on roots of azalea treated with preinoculation and postinoculation applications of metalaxyl. Plant Disease 71:818-820.
Bienapfl, J. C., and Balci, Y. 2014. Movement of Phytophthora spp. in Maryland’s nursery trade. Plant Dis. 98:134-144.
Hamm, P. B., Cooley, S. J., and Hansen, E. M. 1984. Response of Phytophthora spp. to metalaxyl in forest tree nurseries in the Pacific Northwest. Plant Disease 68:671-673.
Hill, S. N., and Hausbeck, M. K. 2008. Virulence and fungicide sensitivity of Phytophthora cactorum isolated from American ginseng gardens in Wisconsin and Michigan. Plant Dis.92:1183-1189.
Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; Corcobado, T.; Cravador, A.; Decourcelle, T.; Denton, G.; Diamandis, S.; Dogmus-Lehtijärvi, H.T.; Franceschini, A.; Ginetti, B.; Glavendekic, M.; Hantula, J.; Hartmann, G.; Herrero, M.; Ivic, D.; Horta Jung, M.; Lilja, A.; Keca, N.; Kramarets, V.; Lyubenova, A.; Machado, H.; Magnano di San Lio, G.; Mansilla Vázquez, P.J.; Marçais, B.; Matsiakh, I.; Milenkovic, I.; Moricca, S.; Nagy, Z.Á.; Nechwatal, J.; Olsson, C.; Oszako, T.; Pane, A.; Paplomatas, E.J.; Pintos Varela, C.; Prospero, S.; Rial Martínez, C.; Rigling, D.; Robin, C.; Rytkönen, A.; Sánchez, M.E.; Scanu, B.; Schlenzig, A.; Schumacher, J.; Slavov, S.; Solla, A.; Sousa, E.; Stenlid, J.; Talgø, V.; Tomic, Z.; Tsopelas, P.; Vannini, A.; Vettraino, A.M.; Wenneker, M.; Woodward, S.; and Peréz-Sierra, A. 2015. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. Forest Pathology. DOI: 10.1111/efp.12239.
Parke, J.L.; Knaus, B.J.; Fieland, V.J.; Lewis, C; Grünwald, N.J. 2014. Phytophthora community structure analyses in Oregon nurseries inform systems approaches to disease management. Phytopathology 104(10):1052-62.
Tjosvold, S. A., Koike, S. T., and Chambers, D. L. 2008. Evaluation of fungicides for the control of Phytophthora ramorum infecting Rhododendron, Camellia, Pieris, and Viburnum. Online. Plant Health Progress doi:10.1094/PHP-2008-0208-01-RS.
Yakabe, L. E., Blomquist, C. L., Thomas, S. L., and MacDonald, J. D. 2009. Identification and frequency of Phytophthora species associated with foliar diseases in California ornamental nurseries. Plant Dis. 93:883-890.