Observations and Comments on Oak and Tanoak Dieback and Mortality in California
Plant Pathologist and Principal
Phytosphere Research, Vacaville, CA
Additional key words: Tanbark-oak, chestnut oak, tanoak sudden death, tanoak decline, sudden oak death, oak decline, Phytophthora ramorum
Previous title: Observations on Tanoak (Lithocarpus
densiflorus) Dieback and Mortality in Marin County, California
Initial release 9/30/99, last
update 11/25/01
Note:
Current information on the biology and host range of Phytophthora ramorum is available from http://www.suddenoakdeath.org and following the associated links from that page. In addition, other studies on P. ramorum canker ("sudden oak death") can be found on the Phytosphere website at http://phytosphere.com/onlinelist.htm.
Phytophthora ramorum causes bark cankers on oak and tanoak
Decline and death of tanoak (Lithocarpus densiflorus) and oak [primarily coast live oak (Quercus agrifolia) but also black oak (Q. kelloggii)] trees have become more common in a number of California coastal counties within the past few years. Some of the earliest reports of unusual tree mortality were from the Mt. Tamalpias vicinity in Marin County (Robertson 1996). The number of affected areas has apparently increased over the past several years, although the rate of increase has not been tracked systematically during this period. The original version of this article was posted in September, 1999, about the time that significant research efforts were being initiated to intensively study the mortality problem.
Beginning in late June 2000, UC Davis plant pathologist David Rizzo isolated a previously undetected pathogenic fungus from affected tanoak, coast live oak and black oak in a number of widely dispersed locations. The agent is a new and rather unusual species of Phytophthora, which has been named P. ramorum. The characteristics of this fungus do not match those of any of the 60 described species of Phytophthora. The fungal growth is favored by relatively cool temperatures - optimum growth in culture occurs near 20 deg C (68 deg F) and growth is negligible at 30 deg C (86 deg F). Sporangia of the fungus are deciduous, a characteristic seen in only two other Phytophthora species, including P. infestans, the cause of potato late blight. This is potentially significant because it suggests the possibility that the sporangia can be transported via air currents. At temperatures somewhat above the growth optimum, sporangia germinate directly by means of a germ tube; at lower temperatures and in the presence of free moisture each sporangium will release a number of zoospores that can swim through water films to seek out suitable infection sites. The fungus has been isolated from symptomatic trees from Napa and Sonoma Counties to the Big Sur area. For the most current data on the confirmed distribution of the new Phytophthora, check the UC Oak Mapper website via the link from http://camfer.cnr.berkeley.edu/oaks.
P. ramorum causes bleeding bark cankers on tanoak, coast live oak and black oak. The cankers are typically found on the lower portion of the trunk but may also occur higher (to more than 12 ft) on the trunk. Cankers have been seen on branches as small as about 1-2 inches in diameter, particularly in tanoak. These cankers are most extensive within the phloem tissues in the bark, but commonly extend to the outer portion of the wood (xylem). Affected xylem tissue typically exhibits a dark, often streaky discoloration; infected phloem tissue is discolored but may appear lighter or more brown than healthy tissue. A dark brown to black line is usually evident at the margins of the infected areas in both xylem and phloem. A reddish to dark brown exudate typically oozes through cracks on the surface of the cankered areas during the wet season. The exudate flow generally decreases or stops during the summer. The fungus has been isolated from infected phloem and xylem tissue and sporangia of the fungus have been observed in the ooze, especially in the spring. Bleeding does not always develop on tanoak cankers, especially in relatively small diameter stems.
Pathogenicity tests have shown that this P. ramorum can cause stem cankers and mortality in oak and tanoak seedlings inoculated in the greenhouse. In these greenhouse tests, tanoak is typically much more susceptible to the fungus than is coast live oak. Field inoculations by Dr. Rizzo in mature trees have produced sizable bleeding cankers within 8 weeks of inoculation. These initial inoculations used pieces of mycelium (the network of microscopic filaments that makes up the 'body' of the fungus) taken from cultures grown in the laboratory. More recently, Dr. Jenny Davidson was able to initiate stem infections in natural field-grown by applying a suspension of sporangia and/or chlamydospores, which is presumably more typical of the type of inoculum that causes natural infections.
P. ramorum also causes leaf spots and/or twig cankers on a number of native plants commonly associated with oaks and tanoak, including California bay, madrone, huckleberry, California buckeye, honeysuckle, bigleaf maple, and other species. It is highly likely that inoculum produced on these hosts is responsible for the stem cankers that occur on live oak and black oak. P. ramorum does cause leaf and twig infections in tanoak, but the degree to which inoculum produced on these surfaces may contribute to stem cankers is uncertain.
Research is underway to address many questions about this disease, including how the pathogen infects trees, the length of time between infection and symptom development, how and where the fungus survives in the environment, how it is transported between trees, and how long the fungus has been present in California. It must be emphasized that the required studies may take some time to complete, partly because P. ramorum is a species new to science, and partly because many of the processes being studied may be slow.
It is still not clear whether the P. ramorum infection alone can kill mature trees or whether tree death normally occurs after infection or invasion by other agents. Based on our own field observations, it appears that stem infections in tanoak may lead to tree death even in the absence of other agents whereas in coast live oak, other agents are almost always involved when trees are killed. As discussed below, the sapwood decaying fungus Hypoxylon thouarsianum and bark and ambrosia beetles are commonly found in P. ramorum-infected trees and may contribute substantially to tree decline. However, these agents are also commonly found on trees that are declining because of other reasons, such as severe decay due to canker rot fungi. Hence, the presence of H. thouarsianum fruiting bodies and/or beetle frass on dead or dying trees is not diagnostic for P. ramorum-related mortality.
The evidence to date suggests that this newly recognized fungus is a primary pathogen of oaks and tanoaks in California, but it is not the only damaging agent affecting oak and tanoaks in California. Oaks have been killed by various well-documented diseases long before the current outbreak was detected. Our research indicates that tree decline and death due to causes other than P. ramorum are common in areas that have high levels of P. ramorum-related mortality. P. ramorum has been associated with bleeding bark cankers on the trunks of tanoak and oaks in the black oak group (which includes Q. agrifolia and Q. kelloggii). However, other agents can also give rise to bleeding cankers on oaks, including other species of Phytophthora that are common in urban landscapes. Many other symptoms found in dead and dying oaks, including wood decay and other types of cankers, are associated with agents other than P. ramorum. Because various pathogens other than P. ramorum can kill mature oaks, it is important to determine the cause(s) of decline and death of affected oaks or tanoaks before taking management actions.
Management implications for homeowners and arborists
Chemical control?
With the public release of information about the discovery of P. ramorum, there has been an increased interest in the use of fungicides on affected or at-risk trees. While it is possible that fungicides may eventually play some role in the management of this disease, it is generally a poor idea for homeowners, arborists, and pest control firms to begin using fungicides on already-infected trees in the absence of any scientific data that supports their efficacy against this disease. There are several reasons for this:
- most established canker diseases in woody tissues are very difficult or impossible to control with fungicides, in part because of the extreme difficulty in getting chemicals into the affected tissues.
- most fungicides inhibit fungal growth or infection but do not actually kill established infections; they are generally fungistatic rather that strictly fungicidal. The chances of eradicating an established fungal infection is woody tissues are extremely small at best.
- most modern systemic fungicides are relatively selective; not all fungicides are effective against all fungi. Some classes of fungicides have activity against Phytophthora species, whereas others are completely ineffective. Fungicide sensitivity also varies among Phytophthora species and isolates.
- host and environmental factors strongly influence disease development; fungicides are generally most effective when they are used in an integrated fashion with other control methods. Attempting to control a poorly understood disease by "spraying and praying" is not only environmentally unjustifiable, but is very unlikely to have any positive effect.
- improper or poorly informed use of fungicides can :
(1) lead to a buildup of fungicide resistance in the target fungi;
(2) have negative impacts on other nontarget organisms, including beneficial
fungi, such as mycorrhizal fungi;
(3) lead to direct plant injury (phytotoxicity).
UC researchers are currently conducting research to determine whether fungicides can play a viable role in the management of this disease in urban areas. See http://www.suddenoakdeath.org for the most current research results.
Current disease management practices
Until the epidemiology of the Phytophthora-related disease complex is fully understood, disease management practices will need to be developed through controlled experimentation. However, some conservative management practices can be adopted in the interim until further information is available:
(a) Because the distribution of the new agent has not yet been established, care should be taken to avoid spreading the fungus into areas where it may not yet be present. The movement of potential host material (including wood and chipped branches, leaves of foliar hosts, field-grown transplants, or nursery stock) and the movement of soil or associated leaf litter from affected areas could spread the disease into additional areas, and should be avoided. Precautions are especially important during the wet season because infection is most likely to occur during the wet season and P. ramorum inoculum (sporangia and zoospores) is likely to be more abundant during the wet season. If affected trees need to be cut down, chipped material and wood should preferably be left on site, and should not be moved into uninfested areas.
(b) Although the mode of infection has not yet been ascertained, wounds favor infection by many fungal pathogens. Within the locations affected by the disease, pruning and other activities that cause wounds to the trunk should be minimized or avoided. Pruning equipment should be disinfested frequently to avoid the possibility of mechanical transmission. Most fungal diseases are more likely to infect during periods when plant surfaces are wet. Hence, pruning during the dry season (especially midsummer) may reduce the likelihood that pruning wounds will be infected. Avoiding wet-season pruning should also help reduce the movement of inoculum between trees.
The first reaction of many homeowners confronted with disease problems in valuable trees is to try to "do something". While this is an natural tendency, there are many more ways to damage and harm trees than there are ways to improve tree health. Hence, "doing something" can often provide no benefit and can make matters worse in some cases.
Replanting in affected areas
A final management issue is the question of replacement. If trees are killed, does it make sense to replant with the same species? At this point, we do not have all of the information needed to give a definitive answer, but we can make a few common sense suggestions based on the known biology and ecology of these systems:
(1) In areas where tree canopy cover is near 100% and stand densities are very high, replacement may not be necessary or desirable if the mortality rate is relatively low. At high stand densities, trees compete with each other for light, soil moisture, and nutrients, and high levels of competition can cause plant stress. Evaluate the density and condition of the stand to determine whether replacement makes sense.
(2) Look for the presence of natural regeneration of oaks or other native species in the understory of the affected trees. Death of overstory trees can reduce the competition that keeps natural seedlings or saplings suppressed in the understory. These understory seedlings can often provide suitable replacements for trees that are lost.
(3) Avoid the temptation to plant the largest replacement tree that you can afford. Large specimens of native oaks or other trees grown in nurseries often have poor root structure and may have soil-borne root diseases (including root decay or cankers caused by other Phytophthora species) or other pests. The chances that a tree will become infected in the nursery generally increase over time, so large stock often has a greater risk of infection than smaller material. Nursery-grown native trees are generally not of a local genotype and may therefore be poorly adapted to local growing conditions. If large trees are transplanted from field grown stock, they also have the additional problem that most of the existing root system is lost in the transplanting process, which severely stresses the tree. In many cases, smaller-sized, locally adapted planting stock will outgrow larger planting stock within a few years and will generally perform better over the long term.
(4) Because we do not know what levels of genetic resistance to P. ramorum exist in native oak and tanoak populations, planting expensive nursery stock of these species is risky at best. However, growing these species on site from direct planted locally collected acorns or from small seedlings grown from local acorns is a viable option. By overplanting and allowing natural selection to occur, seedlings that have some resistance to the pathogen will be the most likely to survive to maturity. This strategy is especially recommended for landowners with large natural stands, although it could even be applied within an urban landscape.
(5) Many of the affected areas support mixed stands of native hardwoods and conifers, including California bay (Umbellularia californica), valley oak (Quercus lobata), bigleaf maple (Acer macrophyllum), California buckeye (Aesculus californica), Douglas-fir (Pseudotsuga menziesii), and coast redwood (Sequoia sempervirens) to name a few. To date, there is no clear evidence that any of these species are killed by P. ramorum although many of these are subject to P. ramorum leaf spots or twig blight. Depending on the site, many of these native species can be used effectively in urban plantings and may be reasonable replacements for affected oaks. Note that comments about using locally-native materials and avoiding large stock sizes applies to these other natives as well. However, the use of foliar hosts (such as bay) is not advised in areas where other susceptible oaks are still present.
Background information, observations, and notes
Because many of the observations made in the original version of this document are still pertinent, I have left the bulk of this article intact for reference purposes. Various sections have been updated on the basis of more recent observations and information from others conducting research on this problem.
Although we have studied disease and arthropod impacts on native oak stands in California since 1988 (Swiecki 1990, Swiecki et al 1990, 1991a, 1991b, 1997), we began to investigate Phytophthora-related mortality in some detail only in September 1999. Prior to that time, I had inspected a few affected Q. agrifolia in urban areas of Marin County. In September 1999, I interviewed a number of people who had investigated and/or observed the mortality problem prior to that time. I also reviewed reports and data which where provided by Dr. Susan Frankel of the US Forest Service. I subsequently made a field inspection of affected sites in Muir Woods National Park and on Marin Municipal Water District (MMWD) lands near Fairfax on 9/22/99, and a follow-up visit to Muir Woods on 2/28/2000. I also participated in an inspection of numerous affected trees on MMWD land on 5/24/00 with a group of plant pathologists and entomologists from UC, CDF, and USFS, and inspected an affected area in China Camp State Park on 7/31/00. In August 2000, we initiated a research project under USFS funding to examine some factors influencing the risk of disease development. We established field plots at 10 locations in Marin County, and one location each in Napa and Sonoma Counties. This research focused primarily on coast live oak (10 of the 12 locations). We completed the field portion of that research by the end of September 2000 and will post the result of that project once we have analyzed the data.
The balance of this report describes a number of my field observations, lab results, and some comments about factors that may contribute to disease problems in oak and tanoak. These observations have been updated since the original release of this report in September 1999 in order to keep pace with the development of addition information about the disease syndrome. Nonetheless, I have maintained references to symptoms discussed in early reports of the disease in an attempt to clarify how the understanding of the disease syndrome has developed over time. The original report included various research recommendations. I have added comments to note where research efforts along these lines are in progress.
Symptoms seen in dying trees
In earlier reports from various sources, various symptoms had been attributed to the decline, which has been called "sudden oak death". These include wilting of young shoot tips, leaf and twig necrosis, cankers on small stems, dark sap bleeding from the lower portion of the trunk, and water-soaking and discoloration of the inner bark and outer sapwood. Basal sprouts or suckers frequently develop at the base of top-killed trees, but it was originally reported that these sprouts typically do not survive long (Svihra 1999). According to early reports, trees of all age classes within an area are typically affected. This is more often seen in tanoak stands than in stands of coast live oak, although we have occasionally seen small diameter (4 inch DBH) coast live oaks with Phytophthora cankers. As noted above, the bleeding cankers of the bark and outer sapwood are probably the most characteristic symptom of the syndrome, and are the symptoms that have been associated with the new Phytophthora species. As discussed below, many of the other symptoms that were originally described as part of the syndrome may be associated with different agents, only some of which may contribute to mortality.
Spatial pattern of affected trees
Affected stands are found across a rather wide geographic area, but so far, reports of the decline syndrome appear to be limited to stands in the Coast Ranges. Within an affected area, the distribution of affected plants is patchy. Individual symptomatic trees can be found sprinkled across the landscape around Mt. Tamalpias and other areas. Patches of affected trees also occur, and the size of individual patches varies. Within most affected areas, asymptomatic trees can be found, but the incidence of disease over a small area can sometimes be quite high.Trees that have died recently are particularly obvious because dead leaves remain attached to the tree and turn a light brown color. Dead leaves can remain attached to dead branches for an extended period, well over six months and possibly more than a year. Visual assessments of disease incidence are biased upward by both the long retention of dead foliage and the fact that affected trees are visually obvious. Although a high proportion of tanoaks in localized areas may be symptomatic, the incidence of disease at the forest level appears to be moderate so far. Statistically valid survey work is needed to ascertain the level of mortality at the stand and forest level. In some areas it may be possible to use aerial photo interpretation or other remote sensing techniques to assess mortality over wide areas. Such work is now underway by UC Berkeley researchers. However, the fact that many of the affected trees are overtopped (discussed below) may limit the usefulness of remote sensing methods in areas such as Muir Woods. Furthermore, remote sensing can only detect dead and dying trees; it cannot be used to determine the cause of tree death. Hence, ground survey methods will continue to be important for assessing the impacts of this disease.
Prior to the discovery of the new Phytophthora, I and others had suggested that the distribution of affected trees was consistent with the hypothesis that one or more native (or long-naturalized) agents may be involved in the disease syndrome. This is still a possibility that must be considered, given that the Phytophthora is only known from coastal California at present. In order for a recently introduced agent to become so widely distributed over such a short time period (i.e., since about 1995), it would have to have several qualities: high mobility (e.g., wind-dispersed) and/or highly efficient and wide-ranging vectors; relatively high levels of inoculum production; and high levels of virulence. Research is still needed to determine if the new Phytophthora possesses these qualities. Mortality centers or disease "hotspots" clearly exist, and our data and field observations indicate that disease incidence varies greatly across relatively small distances. The reasons for this pattern are not entirely clear at present. Quantitative data on this aspect of disease epidemiology will be needed to help model disease progress across the landscape.
We have now looked a fair number of affected stands, primarily in Marin County, but also in Napa and Sonoma Counties. Many of the symptomatic trees that we have observed to date have been growing under conditions where moderate to high levels of plant stress would be expected. Affected trees are commonly either overtopped or in fairly dense stands in which competition for soil moisture and light are likely to be intense. At several sites in MMWD lands, symptomatic tanoak trees were open-grown and widely spaced. However, site conditions appeared to be hotter and drier than is optimal for tanoak, so these trees could also have been under stress. At one site excavated by backhoe on 5/24/00, the affected tree was growing in very shallow soil over a layer of fractured sandstone, which was clearly a drought-prone site. On a portion of one hillside in Muir Woods, stress factors were not immediately obvious. However, close inspection of the area near affected tanoaks revealed a shift in vegetative composition and poor growth in several species, including coast live oak (Quercus agrifolia), Douglas fir (Pseudotsuga menziesii), and manzanita (Arctostaphylos sp.). These observations suggest that the tanoaks in this particular spot may have had low vigor because of low site quality, which may be associated with the soil moisture regime, soil chemistry, or other soil factors. In urbanized areas, it is likely that most if not all affected trees have been subjected to a range of stressful impacts (soil compaction, root damage, altered moisture regimes, etc.).
Based on my early observations, I proposed that decline occurs most commonly in stressed plants, and might even be limited to stressed trees. Various researchers have shown that plant stress can predispose plants to various diseases, including those caused by Phytophthora spp. Local differences in soil type, hydrology, and competition that affect levels of stress could help explain the distribution of the disease, but it is not necessarily easy to quantify these differences. Direct measurements of plant water stress and/or carbohydrate storage are needed to examine relationships between site quality and the incidence of decline. In our recent research, we looked specifically at the role of water stress and various other tree, site, and stand factors on early disease development. We are still analyzing the data and will report the findings of that study in a separate paper.
The patchiness of disease development may also have a genetic component. Tanoak can reproduce vegetatively from burls produced on shallow lateral roots (Burns et al 1990). Therefore, trees in a given localized area can actually be a set of interconnected vegetative clones. Even where trees have arisen from seed, oaks and tanoaks in a localized area will tend to be genetically related because most acorns germinate in the vicinity of the maternal tree. If certain plant genotypes are more susceptible to the agent(s) involved in the decline, or are more prone to become stressed by current stand conditions, we would expect a somewhat patchy distribution of disease symptoms. Finally, it should be noted that individual plants can escape infection due to phenology, vector behavior, uneven inoculum distribution, microclimate, or simply luck. Hence, a spatially uneven distribution of symptomatic plants can arise as the result of one or more unrelated causes, which makes life more difficult for those who are trying to understand the disease syndrome.
Symptoms and agents
Wood-boring insects
Several wood-boring insects that are normally associated with stressed, dying, and/or dead trees have been reported on affected trees. These include oak bark beetles (Pseudopityophthorus spp.) and ambrosia beetles (Monarthrum spp.). Larvae of other wood-boring beetles (e.g., Cerambycidae) have been observed beneath the bark of killed oaks and tanoaks. I have observed evidence of wood boring beetles in some but not all of the dead and dying trees that I inspected. Based on the examination of many trees, it is clear that attack by beetles is not required for tree death to occur, and that the initial development of Phytophthora cankers typically occurs in the absence of beetle attacks. It is likely that many dead trees eventually become infested with wood-boring insects, and the activity of these insects may hasten the decline of at least some trees. However, given that branch and tree death occurs in many trees that have little or no beetle damage, it is unlikely that wood boring insects play a primary role in the decline. No research has been conducted to date that conclusively shows that either oak bark beetles or ambrosia beetles can seriously damage or kill vigorous, healthy oak or tanoak trees. Despite claims to the contrary, studies conducted to date to determine whether tree survival can be extended by treating trees with insecticides have not been conclusive. At least one study conducted by Dr. Matteo Garbelotto has shown no beneficial effect of insecticide treatment. To date, properly controlled and replicated randomized efficacy tests have not been established because of various logistical problems. Such tests, conducted over a sufficiently long time frame, will be needed to demonstrate convincingly whether insecticide treatment has any effect on tree survival.Root symptoms
Other investigators have generally not observed root symptoms on affected plants, although Svihra (1999) noted the presence of Armillaria mellea on a recently-killed tree in 1995. I inspected the root crowns of perhaps 10 declining or dead tanoaks (6 to 8 inch DBH size class) on 9/22/99. I found clear evidence of decay in the root crown area in at least three of these trees which were located in widely separated areas. One symptomatic tree in Muir Woods was heavily colonized by Armillaria (presumably A. mellea); a tree near Bon Tempe Reservoir had a white rot of a buttress root that was not typical of Armillaria and was presumably caused by another basidiomycete; and a third tree near Lake Lagunitas showed incipient decay of the root collar area. On 2/28/00, I inspected a large tanoak that had recently failed at the root crown. This tree had an unusual white rot that had affected one of the major buttress roots and led to the failure. Since the Phytophthora syndrome was not recognized at this time, it is not clear whether these trees were also infected by Phytophthora or were simply declining from root diseases.Although most affected trees sucker from the base after topkill, various observers have suggested that these sprouts generally do not survive long enough to give rise to healthy sprout-origin saplings. This is a topic that needs further study. Based on field observations from 2/28/00, it is clear that basal sprouts from many topkilled trees were still viable at least 6 to 12 months after the death of the top. However, many of these sprouts have been suppressed by deer browsing and shoot tip blighting. The latter symptom is possibly associated with systemic infections by the powdery mildew fungus Cystotheca lanestris, as discussed below.
Given tanoak's propensity to reproduce through vigorous sprouting, the failure of topkilled trees to sprout successfully suggests that root diseases probably play a role in the decline syndrome for at least some trees. We have frequently observed oak declines associate with root diseases in oak stands, and our more limited observations on tanoak suggest that root diseases may be relatively common on this species as well. Root diseases caused by various fungi may therefore contribute to tree mortality in both areas where Phytophthora is present and in areas where it does not occur. The new Phytophthora may or may not be important as a root decay organism. Many Phytophthora species, including most of those in California, cause root decay and/or crown rot symptoms. At this time, it is unknown whether the new Phytophthora species associated with oaks and tanoaks causes root decay. To date, all of the researchers that have examined basal cankers caused by the new Phytophthora have observed that these trunk cankers generally do not extend more than a few inches below the soil surface. This pattern is quite atypical of most Phytophthora species that cause root decay.
Hypoxylon thouarsianum
Some additional confusion about the nature of the decline is related to the initial misidentification of one fungal species that is commonly associated with dead and declining trees. In September 1999, I collected fruiting bodies of a Hypoxylon species that we have identified as H. thouarsianum (Lev.) C.G. Lloyd from tanoak trees in Muir Woods and in the vicinity of the Bon Tempe reservoir. I also collected this fungus from a large canker on a declining coast live oak (Quercus agrifolia) in Muir Woods. We have long noted this fungus in association with cankers on declining and recently killed coast live oak as well as other native oaks (Swiecki et al. 1990). In 1992, we found this species associated with high levels of mortality of interior live oak (Q. wislizeni) at a site in southern Tulare County affected by drought (Swiecki et al 1993). Other reported hosts for the fungus include Q. chrysolepis, Q. douglasii, Q. lobata, and Q. kelloggii (Swiecki et al. 1998). Identification of the species has previously been confirmed by Dr. Isabel Tavares of the UC Berkeley Herbarium. We sent six samples of the fungus from three different hosts (L. densiflorus, Q. agrifolia, and Q. douglasii) to Dr. Jack Rogers (Dept. of Plant Pathology, Washington State University), the world authority on this group of fungi. Dr. Rogers confirmed that all of the samples were H. thouarsianum.
The first image above shows the stroma (fruiting body) of the fungus. The stroma is sliced open to show the carbonaceous interior and the single outer layer of perithecia. The above light micrograph shows the brown, unicellular ascospores and slender asci of the fungus. The asci are apparently evanescent (i.e., they degrade quickly), which is characteristic of H. thouarsianum. We observed them in a section of a fresh stroma collected from a Q. agrifolia in Muir Woods. Only ascospores were visible in older stromata collected from various tanoaks.
The surface of newly-formed stromata may be covered with the conidial or imperfect stage (=anamorph) of the fungus. The conidia are dark olive green and powdery when observed without magnification. Under the microscope, the conidia are subglobose, about 6 microns in diameter, and olivaceous. Conidiophores are densely packed and appear to be sparingly branched. The imperfect stages of Hypoxylon are in the form genus Nodulisporium, but Rogers et al (1999) do not describe the anamorph of H. thouarsianum.
This same fungus has erroneously been identified as a Daldinia species (e.g., Svihra 1999; this error has been corrected in the second printing). Unfortunately, the species is also incorrectly identified and illustrated in David Arora's (1986) book Mushrooms Demystified (p. 887), and this may be the source of the misidentification. Regardless of the source of the error, the differences between Daldinia and H. thouarsianum are of more than strictly taxonomic significance.
Both Daldinia and Hypoxylon cause a white rot of host wood, i.e., they degrade both cellulose and lignin. Daldinia species are primarily saprophytes, although Rogers et al. (1999) note that they may function as weak facultative parasites that continue to decay the wood following decline and death of their hosts. At least in the US, no species of Daldinia is considered to be a significant pathogen. In contrast, most Hypoxylon species range from weak pathogens with high saprophytic capacity (facultative parasites) to virulent pathogens with low saprophytic capacity (facultative saprophytes) (Rogers et al. 1999). The association of H. thouarsianum with cankers on living trees and other field observations suggest that this species can function as a pathogen on at least some hosts, particularly on stressed trees and/or trees attacked by other pathogens.
Many Hypoxylon species initiate latent (dormant or inactive) infections in healthy hosts. These infections can rapidly become active when the host is stressed, and extremely rapid infection and decay of the sapwood can ensue. We originally believed that at least some water-soaking and discoloration of the inner bark and outer sapwood seen in tanoaks and in various oak species was associated with attack by these fungi. As described above, a particular type of bleeding bark canker is associated with the new Phytophthora species. However, these Phytophthora cankers typically do not extend far into the sapwood and do not cause a white rot of the sapwood. It appears that many cankers that involve more extensive decay of the sapwood may be due to infection by H. thouarsianum. Wood that I have associated with H. thouarsianum decay is sometimes discolored, but is generally not as dark as seen in Phytophthora cankers. It may be water-soaked in the early stages of decay, but later becomes dry and light colored. Dark zone lines may be found at the margins of the decayed area. Further research is needed to definitively establish which symptoms are associated with H. thouarsianum .
It is of interest that species of Hypoxylon (sensu lato) have frequently been associated with regional oak declines in the eastern US. It seems likely that H. thouarsianum may play a role in the ongoing decline of oaks and tanoaks, especially given the fact that many affected stands are clearly stressed due to both stand conditions and the presence of another disease agent (new Phytophthora). The buildup of inoculum due to sporulation on numerous dead and declining trees may be increasing the incidence of latent infections within stands. If this is the case, more numerous and larger Hypoxylon cankers may now be developing in stressed trees that would previously have survived. Data on the incidence of latent infections and the epidemiology of H. thouarsianum in tanoak and oak stands are needed to clarify the role of this agent in tree mortality and to determine whether this species needs to be considered in disease management strategies .
Other foliar and stem diseases
From my initial observations of the disease problem on tanoak in the Muir Woods area, I speculated that at least two disease syndromes may be operating simultaneously in some stands - a foliar/stem canker syndrome affecting primarily understory seedlings and small saplings, and a separate trunk/root disease syndrome affecting larger trees. Symptoms of the foliar/stem canker syndrome include:
- partial and complete necrosis of individual leaves, apparently associated with foliar and/or twig infections;
- partial to complete dieback of shoots associated with stem lesions; foliage beyond the lesion dies, sometimes resulting in both dead and live leaves on the same stem;
- cankers on stems of intermediate size (about 1-4 cm diameter); in some cases, these coalesce to girdle and kill the entire stem.
In the branch shown in the image above, the terminal end of the shoot (to the left) is still healthy, but several lateral branches have been girdled by cankers and are dead. The image below shows a stem canker on a small (about 1 cm diam) stem. This canker has not yet girdled the branch.
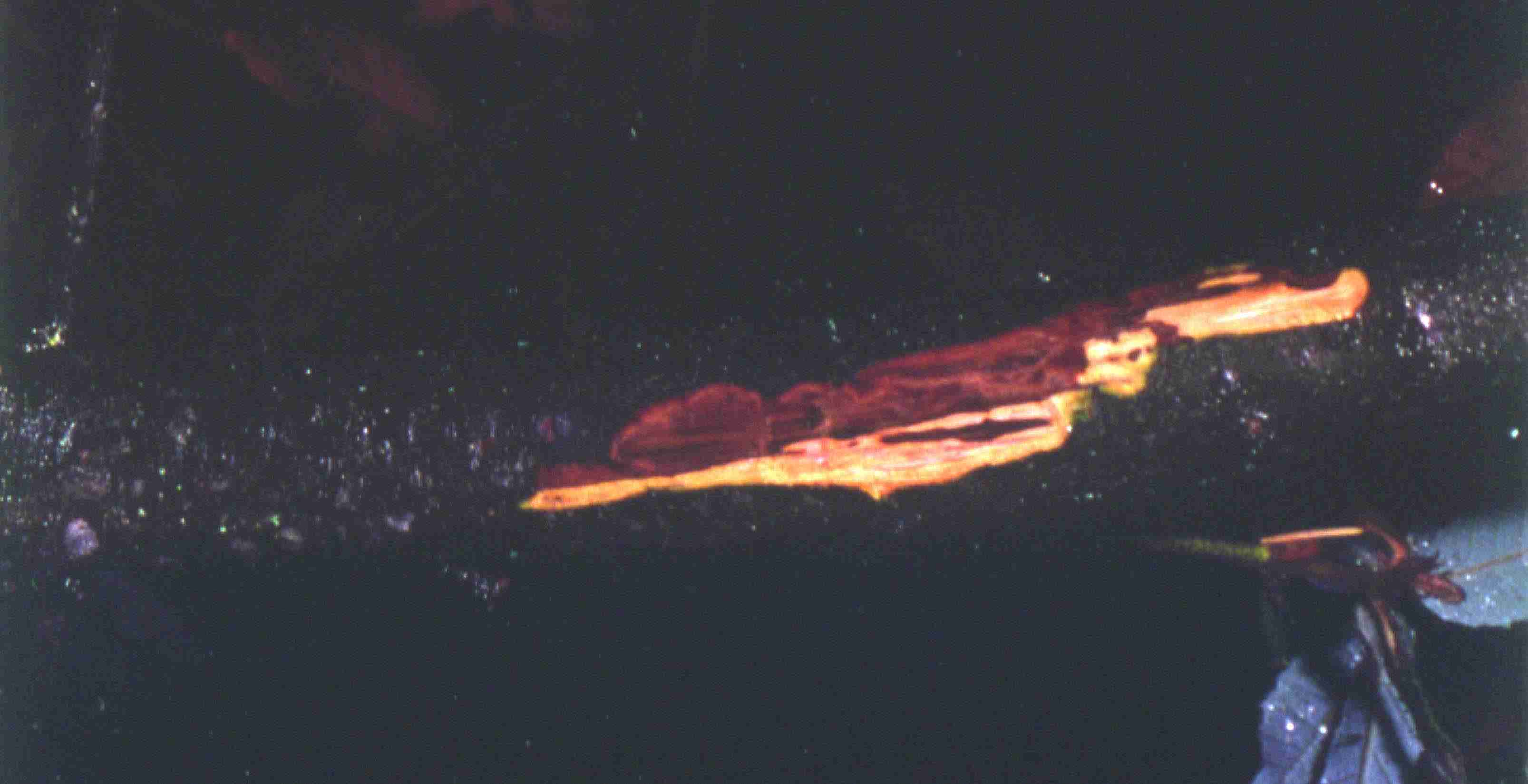
The fungus Diplodia quercina, which causes stem cankers on various California oaks, has previously been identified on stem samples by the CDFA lab. Additional isolations of stem canker fungi, including Diplodia, have been carried out by UC plant pathologists. Although the new Phytophthora has been isolated from stems as small as about 3 cm in diameter, it appears that many of cankers on small diameter stems may be associated with other agents. In my initial investigations, I observed several different fungi associated with necrotic leaf spots as well as two different fungal species associated with dead stems that were at least possible pathogens. In my initial observations, I did not attempt to definitively identify the various fungi, which included both Deuteromycetes and Ascomycetes.
Given that a number of different foliar and stem-infecting fungi attack oaks and tanoaks, it is possible that the assortment of fungi responsible for the foliar/stem canker syndrome may vary from place to place. This possibility is further supported by the variable combination of symptoms on understory seedlings and saplings. Nonetheless, most of these foliar- and stem-infecting fungi have a similar modus operandi and are largely favored by the same types of conditions, i.e., free moisture on plant surfaces. Above-normal rainfall, including significant amounts of rain late in the season, has occurred in most of Northern California in several years prior to the largest dieoffs. These weather conditions have favored foliar and stem canker diseases in both cultivated and native plant populations. For example, unusually severe leafspot and defoliation caused by Septoria sambucina was observed in blue elderberry (Sambucus mexicana) during the 1998 growing season.
At least some of the increase in understory dieback seen in 1999 could be associated with the prodigious 1998 wet season. This would be the case if any of the infections initiated in 1998 had a relatively long latent period and/or if stem cankers initiated in 1998 developed slowly. Furthermore, foliar and stem disease impacts are frequently greater in the understory than in the overstory because inoculum from the overstory is washed down onto understory plants. Disease cycling in the understory can also be aided by summer fog drip from overstory trees in places such as Muir Woods. A 1996 report on the tanoak decline (Neisess 1996) noted that precipitation had been greater than normal for the two years prior to the rash of mortality investigated in that year. The occurrence of favorable disease conditions in several seasons between 1995 and 1999 may have led to an increase in inoculum levels of foliar and stem canker fungi. This could lead to elevated disease levels for several years even if environmental conditions have since become less favorable for disease development. Further observations are needed to determine whether the incidence and severity of canker diseases on small diameter stems (less than 3 cm diameter) and understory seedlings has changed over the past several years.
Svihra (1999) noted various symptoms in root suckers produced by topkilled trees. These symptoms, including bending, chlorosis, and eventual necrosis of the shoot tips, were likened to damage caused by "treatment with phenoxy herbicides". I have also observed these symptom in some though not all basal sprouts of topkilled trees. Affected shoot tips are sometimes thickened and bear abnormally small leaves. In microscopic examinations of fresh bud tissue sections, I have observed intracellular fungal hyphae and haustorium-like structures associated with living host tissues. The macroscopic and microscopic symptoms are consistent with systemic infections caused by the powdery mildew fungus Cystotheca lanestris (Yarwood and Gardner 1972). Like many other powdery mildews, this fungus typically causes severe symptoms on young, rapidly-growing shoots. Positive identification of the causal agent is difficult when affected shoots die back before external sporulation appears. Because powdery mildews are obligate parasites, they will not sporulate on dead host tissue. Further investigation is needed to determine to definitively determine the cause of this symptom, but at this point, this symptom has not been associated Phytophthora infection. Nonetheless, simple wilting of young shoot tips, due to stem girdling or possibly the production of toxins by the fungus, can occur in shoots of trees infected by Phytophthora.
Other agents
Tanoak is thought to represent a link between the chestnut, Castanea, and the oak, Quercus. In fact, tanoak was originally described as a species of Quercus by Hooker and Arnott. Because tanoak is closely related to oaks, it is no surprise that tanoak is attacked by a number of the same pathogens and arthropod pests that attack California oaks. The number of agents that attack tanoak is undoubtedly much less than the over 850 arthropod species and 380 fungal species that have been recorded on oaks in California (Swiecki et al 1997). Nonetheless, we can expect that a goodly number of native arthropods and microorganisms are capable of attacking tanoak.As noted above, I observed decay of the root crown area in some trees. I also observed a few stumps and trees with extensive white rot, such as is associated with Inonotus species and other polypore fungi in the oaks. I also observed Ganoderma basidiocarps on associated tree species, especially California bay (Umbellularia californica). Several Ganoderma species cause root rots of California oaks, and can result in the apparently rapid collapse of trees that do not show chronic symptoms of decline. Other investigators have not reported much evidence of wood decay other than sapwood decay and discoloration, which is sometimes restricted to sectors of the stem when seen in cross section (Susan Frankel, personal communication). The latter symptom could be due to canker rot fungi such as Inonotus, which are common pathogens of California oaks. In our recent field research, we have documented moderate to high levels of canker rot infection in coast live oak stands that also have Phytophthora-related mortality. We also observed symptoms typical of those caused by canker-rot fungi in large-diameter tanoaks.
Overall, basidiomycete wood decay fungi have been the most important pathogens in native oak stands in California, so it would be somewhat surprising if wood decay fungi were uncommon in older tanoak stands. Past stand management and stand age can greatly affect levels of decay in older trees, so levels of decay may vary greatly between stands. Where relatively even-aged stands have developed following clearing, decadent trees that support wood decay fungi may be relatively uncommon. As a result, such stands may have levels of wood decay lower than would be expected in natural mixed-age stands. More data is needed to determine how much mortality of oak and tanoak is associated with wood decay pathogens across the range of tanoak. Our limited data on tanoak (2 locations, 22 plots) suggests that Phytophthora-related mortality in these stands may be of a much greater magnitude than mortality associated with wood decay or other native agents. In contrast, in the 10 coast live oaks stands we investigated (128 plots), decline and mortality associated with Phytophthora infection was somewhat less common than decline and mortality associated with canker rots and other wood decay fungi. This is especially significant because these locations were sampled on the basis of having high levels of Phytophthora infection. The point is that wood decay fungi continue to be primary agents associated with decline and mortality in mature oak trees in many areas. It can be somewhat difficult to associate wood-decaying pathogens with decline because they cannot be isolated with standard techniques and some (e.g., I. andersonii) may not fruit on affected trees until they have been dead for an extended period.
The discovery of the previously undetected Phytophthora on oaks and tanoaks adds to the list of known pathogens; it does not remove other well known pathogens from the list. Nonetheless, if Phytophthora disease incidence remains high, it could supplant wood decay and canker rot fungi as the most important pathogen of coast live oak and black oak. Because the decay fungi are generally very slow acting, trees infected by Phytophthora may be killed by this agent and its associated disease/pest complex long before they could be killed by canker rots or other wood decay fungi.
Forest conditions contributing to decline
If the new Phytophthora turns out to be a recent introduction, the timing of the epidemic will have a fairly simple explanation. However, if it turns out that this fungus has been present in California for an extended period, we will need to ask why these stands are being affected now. I have already discussed the fact that weather conditions have been somewhat unusual during the period that the problem has been observed. The conditions - several seasons with high rainfall followed by a year with an early end to rainfall, leading to late season water stress - could be highly favorable for the spread and disease development by a Phytophthora species whether it was native or introduced.
In addition, we need to bear in mind that stand conditions have been changing over time. Stand composition, density, and stratification are probably much different now than they have been at any time in the past century. Virtually all of these stands have been greatly altered by human activities that were initiated with the settlement of California. Most stands have been affected by episodes of logging of conifers and/or hardwoods, destruction of mature tanoak stands through stripping of bark for tannin, alterations in fire frequency and intensity, and/or changes in vegetative composition and structure. In Marin County and many other areas, cutting and other more intensive activities ceased by the early decades of this century. As a result of continued growth and a lack of other stand-thinning events, such as fire, the second and third growth stands that constitute much of the oak and tanoak resource may have reached a stage in which competition has become critical. Even in the absence of a new pathogen, these stands were reaching a stage where the likelihood of widespread decline and mortality were increasing.
As noted by Skelly and Innes (1994), declines in natural forests affecting one or more related species are fairly common events in both North America and Europe. These declines typically involve a suite of agents and site and environmental conditions. In many of the oak declines that have affected the eastern US, affected trees are typically stressed by one or more biotic or abiotic factors and are subsequently killed by various opportunistic agents (Kessler 1989).
Even though the new Phytophthora has been associated with in the early stages of oak and tanoak decline, it is clear that this agent is not solely responsible for all recent mortality in oak or tanoak. The distribution of disease and tree decline is likely to be related to a complex of interacting factors, including the current stand conditions, environmental conditions, and an array of secondary and auxiliary pathogens and insects. The overuse of the term "sudden oak death" as led to an oversimplification of a rather complex disease situation. Casual observations are not sufficient to document the presence of Phytophthora-related mortality in an area. Actual detection of the Phytophthora is needed to confirm that tree decline or even bleeding bark canker symptoms can be attributed to this agent, especially in areas that are beyond the known range of the pathogen.
In many of the affected stands, trees are already growing under stressful conditions (e.g., high levels of competition and shading), have latent infections of opportunistic pathogens such as H. thouarsianum, and may have substantial amounts of root or trunk decay. Under such conditions, any additional stress may be sufficient to tip the balance in favor of disease development and cause a relatively rapid plant decline. Phytophthora infection or a heavy episode of defoliation and stem dieback due to other stem canker fungi could provide the additional stress that triggers massive attacks by fungi such as H. thouarsianum and by bark and ambrosia beetles. In other words, at least some of the trees that have died during the last few years might well have died anyway within the next 10 to 15 years. Unusual weather conditions may have set off a cascade of events that have helped to synchronize some of this mortality over a relatively wide geographic area. Furthermore, once the decline has started in an area, the buildup of fungal inoculum and insect populations on dead and dying trees provides additional pressure that may accelerate the decline of trees that might resist a lower intensity of attack.
Conclusions
Clearly, there are still many questions to be answered about the unusual mortality affecting oak and tanoak in the Coast Ranges. It may take some time to thoroughly investigate this problem because very little research effort has been directed toward the understanding of oak or tanoak pests and diseases to date. Tanoak is not only a largely noncommercial species (some commercial harvest does occur), but it is considered a weed in many commercial conifer stands. Nonetheless, tanoak and oaks are an important component of various forest types in the North Coast Ranges of California and the urban forests of Marin and other coastal counties. Public agencies and private landowners need sound information to both understand the impacts of the decline on their stands and determine what, if any, actions can or should be taken.
The current disease situation has not developed overnight, and its consequences will continue for years. Although research efforts are now being directed towards oaks and tanoaks in affected areas, a much broader ongoing effort is needed to manage California's critical oak and hardwood-dominated ecosystems. In our 1990 report to CDF (Swiecki et al 1990), we made the the following case for monitoring the health of oak woodlands:
"However, further monitoring of disease impacts through establishment of additional survey plots would provide the following benefits:
- permit further analysis of the geographic distribution of disease impacts
- improve estimates of the proportion of the oak resource affected by decay
- expand observations to other oak species
- allow for further analysis of environmental and cultural factors associated
with disease severity and mortality
- establish a broader baseline for use in future evaluations of trends occurring
over time.
The groundwork already established through our project should facilitate the future collection, storage, and analysis of data on the condition of oak stands. An additional benefit of expanded and continued monitoring would be the early detection of exotic pests and diseases that could severely impact the oak resource. Among these, oak wilt, caused by the fungus Ceratocystis fagacearum, and the gypsy moth, Lymantria dispar, stand out as two agents that have been particularly destructive to oaks in the eastern U.S. These agents are currently quarantined. Trapping has been used routinely to detect accidental introductions of the gypsy moth, but early detection of oak wilt would rely strictly on diagnosis of affected trees. Since it is not possible to completely exclude every potentially damaging exotic agent, continued monitoring of oak health would provide a means for detecting and hopefully minimizing the effects of these agents."
Hopefully, the current problems with oak and tanoak will prompt state and federal authorities to look seriously at developing a proactive, integrated strategy for the management of California's oak woodlands and other noncommercial but ecologically critical woodlands and forests.
Acknowledgments
I would like to thank the following persons who provided information about their observations and/or provided access to field areas: Mia Monroe (NPS), David Minkler (Arborist and NPs volunteer), Dr. Dennis Odion (MMWD), Dr. Susan Frankel (USDA-FS), Dr. David Adams (CDF), Dr. Pavel Svihra (Marin Co. UCCE), and Dr. David Rizzo (UC Davis). Dr. Elizabeth Bernhardt of Phytosphere Research provided editorial input to this article and is a coinvestigator in our recent USFS research project.References
Burns, Russell M., and Barbara H. Honkala, tech. coords. 1990. Silvics of North America: Vol 2. Hardwoods. AgricultureHandbook 654. Washington, DC: U.S. Department of Agriculture, Forest Service. Web site: http://willow.ncfes.umn.edu/silvics_manual/Table_of_contents.htm
Kessler, K. J. 1989. Some perspectives on oak decline in the 80's. p. 25-29 in Proceedings of the 7th Central Hardwood Forest Conference. Gen. Tech. Rep. NC-132. St. Paul MN: US Department of Agriculture, Forest Service.
Neisess, J. S. 1996. Letter to Dennis Odion, Marin Municipal Water District, dated 9/12/96. File code 3420. San Francisco: USDA Forest Service, State and Private Forestry. 2 pages.
Robertson, A. (editor-in-chief) 1996. Forest pest conditions in California - 1996. California Forest Pest Council.
Rogers, J. D.; Ju, Y.; Adams, M.J. 1999. Home of the Xylariaceae. Web site: http://mycology.wsu.edu/xylariaceae/default.asp
Skelly, J. M.; Innes, J. L. 1994. Waldsterben in the forests of Central Europe and Eastern North America: Fantasy or Reality? Plant Disease 78:1021-1032.
Svihra, P. 1999. Sudden death of tanoak, Lithocarpus densiflorus. Pest Alert 1. June 1999. San Rafael: UC Cooperative Extension, Marin Co.
Swiecki, T. J. 1990. Oak diseases and insects: a delicate balance. Fremontia 18:58-63.
Swiecki, T. J.; Bernhardt, E. A.; Arnold, R. A. 1990. Impacts of diseases and arthropods on California's rangeland oaks. Prepared for CDF Forest and Rangeland Resource Assessment Program, Sacramento, CA.
Swiecki, T. J.; Bernhardt, E. A.; Arnold, R. A. 1991a. Insect and disease impacts on blue oak acorns and seedlings. Pages 149-155 in the Proceedings of the Symposium for Oak Woodlands and Hardwood Rangeland Management. Gen. Tech. Rep. PSW-126. USDA Forest Service Pacific Southwest Forest and Range Exp. Stn., Berkeley, CA.
Swiecki, T. J.; Bernhardt, E. A.; Arnold, R. A. 1991b. Monitoring insect and disease impacts on rangeland oaks in California. Pages 208-213 in the Proceedings of the Symposium for Oak Woodlands and Hardwood Rangeland Management. Gen. Tech. Rep. PSW-126. USDA Forest Service Pacific Southwest Forest and Range Exp. Stn., Berkeley, CA.
Swiecki, T. J.; Bernhardt, E. A.; Arnold, R. A. 1997. The California oak disease and arthropod (CODA) database. Pages 543-552 in the Proceedings of the Symposium on Oak Woodlands: Ecology, Management, and Urban Interface Issues. Gen. Tech. Rep. PSW-160. USDA Forest Service Pacific Southwest Forest and Range Exp. Stn., Albany, CA.
Swiecki, T. J.; Bernhardt, E. A.; Arnold, R. A.; Kellogg, J. 1998. CODA - California Oak Disease and Arthropod Host Index Database. Data release 10/98. Available for downloading from http://phytosphere.com/phytosp3.htm.
Swiecki, T. J.; Bernhardt, E.; Drake, C. 1993. Factors Affecting Blue Oak Sapling Recruitment and Regeneration. Prepared for CDF Strategic Planning Program, Sacramento, CA.
Yarwood, C.E.; Gardner, M.W. 1972. Powdery mildews favored by man. Plant Disease Reporter 56:852-855.
Note: This copyrighted report is available exclusively online, and as such may be revised from time to time. If you have comments or questions about this report, please let us know. You can e-mail us at Phytosphere@phytosphere.com