Using green pears to bait for Phytophthora in soil / root samples
Elizabeth Bernhardt and Ted Swiecki, Phytosphere Research
version 12/23/2019
Information on using green pears as baits to detect Phytophthora is contained in two pages. See 3.2. Detection by baiting - general procedures and details for water samples for the following topics:
- Background
- Selecting and preparing baits
- Baiting irrigation leachate and other water samples
- Disposing of infected baits
This document discusses
- the use of green pears to bait Phytophthora from soil/root samples
- symptoms of Phytophthora infections on pear baits
- pear symptoms caused by agents other than Phytophthora
- methods for confirming that bait lesions are caused by Phytophthora
See Testing Procedures for BMPs for Producing Clean Nursery Stock for additional information on specific baiting protocols for nursery stock.
Baiting soil / root samples
In general, processing of soil/root samples should begin within a day of sample collection. Once roots have been cut off and damaged in the sampling process, they are subject to drying out and attack by many organisms besides Phytophthora. Storing samples at cold temperatures (4 C) has also been shown to reduce recovery of at least some Phytophthora species from root/soil samples, so it is preferable to collect soil/root samples when they can be processed as quickly as possible, rather than collecting samples and storing them in a refrigerator until some later date.
Preincubation of dry or cold samples
For dry samples: If the sampled soil has been dry for an extended period, sporangia may not be present or may be present in only small numbers. Because the presence of sporangia is critical for the success of baiting, detection of Phytophthora can be enhanced by adjusting the soil moisture to field capacity and preincubating the soil at room temperature (about 18-24 C [65-75 F]) for 72 hours before baiting to promote sporangium production.
The amount of water needed to adjust the soil to field capacity (moist but not at all saturated) will vary with the volume of soil, its moisture content, structure, and texture. Add water slowly and mix the sample by gently massaging the plastic bag it is in to avoid overwetting the soil. Avoid using pressure in this process if the sample contains sharp objects that may poke holes in the sample bag. If hard soil clods are present, it may be necessary to apply water and wait for at least 15 minutes to allow the water to penetrate and soften these aggregates. In general, it is better to initially wet the soil to somewhat less than field capacity, wait a few hours, and add additional water later if needed. You can use a spray bottle to add small increments of water and to make sure that root fragments that stick out of the soil are moist.
During the preincubation period, allow the samples to have some air exchange but not dry out. For plastic zip closure bags, the bags should be left unsealed, but the tops folded over lightly to help maintain humidity. The samples should be checked daily and misted with water and/or agitated to make sure that the surface roots and soil do not dry out. After the 72 hour preincubation period, pear baits are added and the samples are flooded as described below.
For cold samples: Sporangium production by many Phytophthora species may be limited at cold temperatures. Even if the soils are moist, preincubation of soil/root samples at room temperatures may be helpful if the sampled soils have been at average temperatures below 10-15 C [50-59 F] for an extended period. If necessary, add water to moisten cold samples to near field capacity as noted above, then preincubate at room temperature (about 18-24 C [65-75 F]) for 72 hours to promote sporangium production before flooding soils and adding pear baits. If the samples are saturated at the time of collection, zoospores could be present and the samples should be baited immediately after collection. As for dry samples discussed above, allow for air exchange during preincubation but prevent the surface and exposed roots from drying out. It may be necessary to mist the samples periodically with water.
Baiting soil/root samples
Samples from a moist site (e.g., irrigated nursery stock, field site with moist soil near field capacity) where soil temperatures have not been cold (as described above), can be baited and flooded immediately as described below.
If samples are collected in heavy duty 1-gallon zip-closure bags (e.g., 1-gallon Freezer Ziploc bags), preincubation (if needed) and baiting can occur in the same bag, reducing sample processing time and possibilities for cross-contamination ( Figure 1). If holes develop in the sample bag from rocks or woody roots, the sample should be overbagged with a second bag before water is added to flood the sample. Overbagging can also be conducted if a leak is detected after the sample is flooded, but this is generally more difficult. After baiting is completed, excess water can be decanted and the sample bags can be sealed for disposal. Plastic bags simplify processing and cleanup, but once the samples are flooded, bags need to be supported to keep the edge of the plastic bag above the water level and help keep the pear in position. The container will also serve to contain spills or leakage from the bags. For flooded soil samples in 1-gallon (26.8 x 27.3 cm) bags, a container with sides about 11 cm high is adequate.
Alternatively, samples to be baited can be placed in clean containers as shown in Figure 3. New containers should be washed with dilute detergent and well rinsed to remove chemical residues before use. Reused containers should be completely cleaned, sanitized, and thoroughly rinsed before use. Phytophthora zoospores have been reported to swim up to about 7 cm through still water, and smaller distances through saturated soil, so there is no advantage to using overly large containers or samples.
Pears can be placed upright or tilted at about a 45 degree angle, which increases the contact area at the waterline. Keep the stem above water if possible. If the sample includes hard or abrasive items (e.g., sand grains, perlite, rock fragments, large woody roots), the pear should be inserted very carefully into the sample to avoid causing wounds. If the sample is in a plastic bag, you can use your hand from the outside of the bag to create a depression in the soil where the pear will rest. In a typical soil/root sample, about 2 to 3 cm of the pear will rest in the depression in the soil. If possible, orient the pear so that any wounds present will be above the water line of the flooded sample. After the pear has been placed in the sample, add enough water to flood the sample to a depth of about 2 cm above the soil level. Water should be at room temperature or cooler and should have little or no chlorine. You can use carbon-filtered tap water, bottled drinking water, distilled water, or tap water that has been boiled and cooled or has been aged in an aerated container for 24 hours to allow chlorine to dissipate.
Incubation temperatures with diurnal fluctuations from 18-24 C (65-75 F) are generally suitable for detecting a variety of Phytophthora species using pear baits. Phytophthora species prefer well-aerated conditions, so leave bags open on top to allow for air exchange. Leaving bags open will also reduce ethylene accumulation, which speeds ripening of the pear baits.
Zoospore release from existing sporangia may start within the first hour after the samples are flooded. If mature sporangia are not present, zoospore release may be delayed for up to several days. If sporangia continue to be produced in the sample, zoospore release may occur over an extended period. Secondary cycling may also occur within the flooded sample; root fragments may serve as baits that become infected and subsequently produce sporangia and zoospores. This process can help increase inoculum levels to improve detection efficiency. To allow time for these processes, pear baits are left in flooded samples for up to 5 full days. Inspect the flooded samples daily to make sure that the bags are not leaking and to check for symptom development.
In very highly infested samples, Phytophthora symptoms are sometimes visible after samples have been flooded for as little as two days, but more commonly, the first Phytophthora lesions are not evident until at least three days after flooding. When checking for symptoms during incubation, it may be necessary to use a clean waterproof glove to carefully pick up the pear and check the bottom. Be careful to contain all drips in the sample bag, and gently replace the pear after inspection if it lacks obvious symptoms. It may be helpful to gently redistribute the soil sample somewhat at this point. This can be done by manipulating the outside of the sample bag while holding or supporting it. The objective is to free up or redistribute sporangia that may be trapped at the bottom or corners of the bag. Be careful to avoid cross contamination between samples when handling pears. Wash and sanitize gloves with alcohol (70% isopropanol) between samples, making sure to rinse of all soap alcohol residues before handling another bait.
Remove the pear bait from the sample as soon as distinct Phytophthora lesions become visible. Note the date and time of removal for each bait. If the pear is left in the water after lesions have appeared, these necrotic areas can become more vulnerable to secondary infection by other organisms, which complicates diagnosis and confirmation. You may also need to remove pears if they have become heavily infected by Pythium or have developed epidermal cracking (discussed below, Figure 9). Remove all pears from flooded samples after 5 full days, even if no lesions have appeared. In some situations, lesions become visible a day or two after the pear has been removed from the flooded sample.
As described above, use clean gloves to carefully remove each pear bait and rinse it under cool running tap water to remove soil and debris. Be careful to avoid cross-contamination of other samples from splashing water. Avoid holding the pear where lesions are visible, since these lesions may rupture under pressure. Pears may develop a slippery biofilm on submerged surfaces; this does not have to be rinsed off completely. Set pears to dry on clean racks or paper towels, spaced so they do not touch each other. Keep them indoors at room temperature (between about 18-24 C [65-75 F]). If Phytophthora symptoms are not visible when the pears are removed, continue to observe the pears for at least 3 (and up to 5) days for delayed symptom development. A standard test requires at least 8 days from the start of baiting before a negative result (no detection) is recorded. However, if pears are overrun with Pythium infections, the pear may become too decayed to continue observations for this whole period. Keeping the pears at the cooler end of the temperature range can help slow expansion of some Pythium lesions.
After baits are removed from the flooded samples, inspect the pears at least twice a day to check for lesion development. Additional lesions on infected pears may develop one or more days after the first lesions. A single pear bait may have lesions from several different Phytophthora species that were present in the sample. Lesions caused by Phytophthora and Pythium will continue to expand and coalesce. In general, this process will occur faster at warm temperatures.
 Figure 1. Overhead view of pear baits in heavy duty 1-gallon (26.8 x 27.3 cm) zip closure bags. Samples of this size have good detection sensitivity and are relatively easy to collect and process.
Figure 1. Overhead view of pear baits in heavy duty 1-gallon (26.8 x 27.3 cm) zip closure bags. Samples of this size have good detection sensitivity and are relatively easy to collect and process.
Figure 2. Baiting a large number of small samples using 1 quart plastic bags.
Figure 3. Baiting a large soil / root sample in a plastic bin. Two baits are used to increase the detection efficiency in this large sample.
Interpreting symptoms on baits
Phytophthora infected pear baits can show different types of symptoms depending on:
- the pear variety used as bait and how green it is;
- the time elapsed between infection and observation;
- the species of Phytophthora causing the lesion;
- the amount of Phytophthora inoculum in the sample.
All these factors need to be considered when interpreting pear lesions and accounting for differences in symptom expression. The pears in Figure 5 were all removed from a series of soil samples at the same time and show strong differences in lesion development due to variable amounts of Phytophthora cinnamomi inoculum in the samples.
Most Phytophthora species cause medium to dark brown lesions on pears that are initially firm and mostly confined to the epidermis, with little or no softening of the underlying fleshy tissue (mesocarp). Initially, the mesocarp under the lesion will not be brown. One major exception to this general appearance is that some Phytophthora species, especially those in Phytophthora clade 6, produce light-colored lesions that have variable amounts of brown flecking (Figure 7). However, even these lesions are relatively firm initially compared with Pythium lesions. After a few days, most Phytophthora lesions will become softer and somewhat sunken and may develop a water-soaked appearance near the edges.
Phytophthora infections can occur anywhere on the submerged portion of the pear bait. They are commonly initiated at lenticels on the pear surface but can also occur at old or fresh wounds or sometimes at the edge of the grocery sticker. If the amount of Phytophthora inoculum in the sample is very low, isolated lesions may form, often at the water line or on the bottom of the pear where it was in contact with the soil or roots. If inoculum levels in the soil sample are higher, but still relatively low, a "bathtub ring " of lesions will commonly form at the water line (figure 4). This is the result of infections initiated by zoospores that swam to the water surface and infected the pear at that level. If the pear was shifted during incubation, it may develop more than one such ring of water-line lesions. In samples with high amounts of Phytophthora inoculum, much of the submerged portion of the pear bait may be covered with brown lesions within 3 days after the sample is flooded (figure 5). These lesions can merge quickly, so the affected part of the pear may turn completely brown (figure 5).
The brown color of Phytophthora lesions appears to involve oxidation of affected tissues. Portions of pear baits that are submerged in water sometimes may initially only develop subtle light discolored patches. When removed from the water and exposed to air, such lesions will darken quickly, commonly within an hour).
The suite of symptoms noted above, especially firm, dark brown lesions that develop in nonwounded tissue such as in figure 6, top row) or in the "bathtub ring" pattern are rarely, if ever, due to anything other than Phytophthora. However, some Phytophthora lesions can fall outside of this "classic" symptom set and may be more difficult to interpret. Interpretation can be further complicated if symptoms caused by other agents (described below) are present. Small Phytophthora lesions can also sometimes be overrun by fast-expanding Pythium lesions, which is why baits should be inspected frequently. Confirmation that lesions are caused by Phytophthora species requires lab tests, some of which are described briefly in the last section of this document.
Symptoms caused by Pythium and other organisms
For convenience, we have referred to Pythium in its historical wide sense (sensu lato or s.l.). This group of water molds is closely related to Phytophthora and has long been noted to consist of several distinct groups. Various authors have subdivided Pythium s.l. into several genera, including Phytophytium and Pythiogeton, or alternatively into Ovatisporangium, Globisporangium, Elongisporangium, and Pilasporangium. Some Pythium s.l. are strictly saphrophytes that decay dead plant material. Others are opportunistic or weak pathogens, especially of seedlings, whereas others are more aggressive pathogens.
Pythium species commonly infect pear baits, but can only infect at a wound, which is usually conspicuous near the center of the lesion. This is why it is important to use only intact pears that are as free of wounds as possible as baits. Pythium lesions in pears are initially soft and translucent or watersoaked in appearance and remain this way as they expand over time. They commonly become sunken very quickly and the fleshy pear mesocarp under the surface of the lesion will be brown. Because of the diversity of species in Pythium , lesions caused by these water molds are variable. Some Pythium lesions expand rather quickly, whereas others progress much more slowly and may eventually stall out. Some Pythium lesions may appear to be somewhat firm, but when the epidermis over the lesion is peeled back, soft decay of the underlying pear mesocarp is evident. Pythium lesions may be light to dark brown in color
Some true fungi can also cause lesions on pear baits, though these are less common than Pythium lesions. These lesions are also typically initiated at wounds and become sunken very quickly. In contrast to Pythium lesions, lesions caused by fungi are typically opaque, not translucent. Often these lesions become limited in size and start to dry out and crack after a few days. Fungal lesions occasionally develop at the stem end of the pear, but pears with this condition can usually be avoided by careful selection of baits.
Abiotic symptoms
Pear baits can develop some symptoms that are apparently due to factors other than microoganisms. Unlike lesions caused by biotic agents, lesions or discoloration caused by abiotic factors do not continue to expand over time once the pear is removed from the sample.
Submerged portions of some pear baits incubating in water or flooded soil/root samples develop a network of splits or cracks, sometimes starting at wounds (Figure 9). This epidermal cracking appears to be related to uptake of water through the epidermis and subsequent swelling of underlying cells in the mesocarp. The symptom occurs sporadically and occurs more commonly in water samples than soil/root samples. In some cases, it appears to be related to unusual water chemistry (e.g., high acidity), but also seems to be related to the postharvest physiology of particular pears. These cracks are fresh wounds that can be colonized by Pythium or other organisms, so they may later show expanding lesions associated with these secondary infections. Pear baits should be removed from water immediately if substantial cracking develops, as they will only become more degraded if they remain in water. Cracks that are not infected become sunken over time as they dry out.
In some instances, water and soil chemistry can cause surface discoloration of the pear epidermis that is limited to the water contact area. The discolored area will be firm but will not expand further once the pear is removed from the water (Figure 11). This uncommon pear reaction may interfere with the test, especially if it is extensive. If the test is negative or difficult to assess because of such symptoms, conduct a new test if possible.
Epidermal pitting (figure 10) is another uncommon, presumably abiotic symptom we have observed in some baits from water samples. It appears to be related to infiltration of water into lenticels and subsequent collapse of underlying cells.
Scald (Figure 12) is a known postharvest disorder of pears that have been stored under improper conditions. The firm, brown surface discoloration is superficially similar to Phytophthora lesions, but does not show the typical distribution of Phytophthora lesions and does not expand over time. Do not select pear baits that show such symptoms or that come from a batch of pears with these symptoms, because scald symptoms may continue to appear after the baits are placed in the sample, complicating assessment.
Figure 4. "Bathtub ring" of Phytophthora infections that were initiated near the water line of pear baits. Lower image is of a pear that was tilted during baiting.
Start checking the pear for symptoms after 2 to 3 days. If necessary, carefully pick up the pear and check the bottom, being careful to contain all drips in the sample bag, and gently replace the pear after inspection if it lacks obvious symptoms. It may be helpful to gently redistribute the soil sample somewhat at this point. The objective is to free up or redistribute sporangia that may be trapped at the bottom or corner of the bag or container. Be careful to avoid cross contamination between samples when handling pears. If possible use gloves and wash hands/gloves between samples with soap and water or rinse with alcohol (70% isopropanol) and then water (to avoid dripping alcohol into the samples).
Remove pears after 5 days, even if no spots have shown up yet. In some situations, lesions may become visible a day or two after the pear has been removed from the flooded sample.
Once lesions become visible, remove the pear and rinse it well with tap water at a sink. Be careful to avoid cross-contamination of other samples from splashing water. There is no advantage to leaving the pear in the water once lesions appear, as these necrotic areas will become more vulnerable to infection by other organisms, complicating diagnosis. Set pears to dry on racks or paper towels so they do not touch each other. You can keep them at the same temperature range used for baiting. Spots will continue to grow in size and coalesce. In general, this process will occur faster at warm temperatures. The pears in the image below were all removed from a series of soil samples at the same time, and show strong differences in infection due to variable amounts of inoculum in the soil.
Figure 5. Bartlett pears exposed to varying levels of Phytophthora cinnamomi inoculum in soil samples. Top two rows show decreasing levels of inoculum from left to right. The three pears at the bottom were incubated in soil samples that were negative for the presence of Phytophthora. Phytophthora cinnamomi was isolated from lesions on all the pears in the top two rows in the image above. Note the dotted lines on some pears, which encircle various wounds or imperfections seen before baiting.
Phytophthora forms dark brown, initially firm lesions on pears. The lesions will become softer over time and can develop a water soaked appearance near the edges. Much of the submerged portion of the pear bait may be covered with brown lesions within 2 to 3 days after being placed in the flooded sample if the sample has high amounts of Phytophthora inoculum. These lesions can merge quickly, so the affected part of the pear may turn completely brown. If the amount of Phytophthora inoculum is low, isolated spots may form, often at the water line or on the bottom of the pear where it was in contact with the soil or roots.
Observations of brown lesions on unblemished green pears strongly suggest that the sample contains Phytophthora species. Lesions that start out soft and watersoaked are commonly associated with Pythium infections or various fungi. Although "bathtub rings" of lesions are almost always due to Phytophthora infections, individual spots are more difficult to interpret. The appearance of the lesion can be a good indicator, but is not definitive. To confirm the pears have been infected by a Phytophthora species, it is necessary to confirm the presence of the pathogen through further testing.
Figure 6. Lesions on D'Anjou pear baits caused by Phytophthora cinnamomi (top) and Pythium species (bottom).
Figure 7. Lesions on D'Anjou pear baits caused by different Phytophthora species. Lesions on both pears are firm but those on the right (P. riparia) are light-colored with only flecks of dark brown discoloration.
 Figure 8. Lesions on D'Anjou pear baits from a water sample caused by Phytophthora cambivora at two different zoospore concentrations. Lesions will darken further over time.
Figure 8. Lesions on D'Anjou pear baits from a water sample caused by Phytophthora cambivora at two different zoospore concentrations. Lesions will darken further over time.

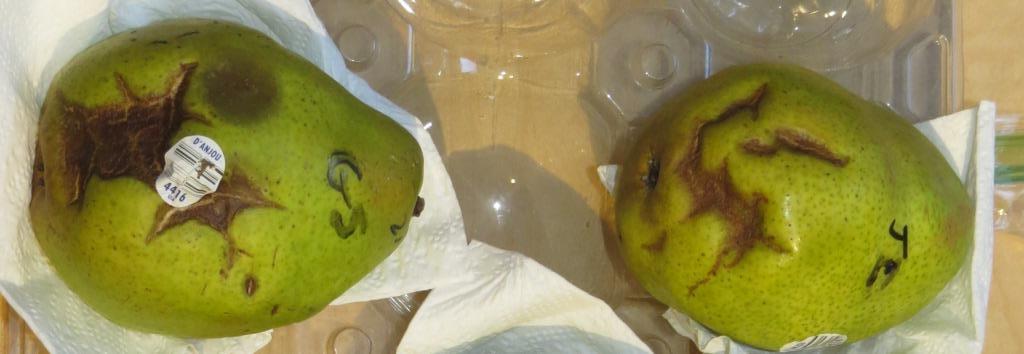 Figure 9. Epidermal cracking symptoms in D'Anjou pears. Symptoms in the upper photo developed after prolonged refrigerated storage of the pears in a sealed plastic bag; these pears were not used for baiting. In the lower photo, cracks developed during incubation in water samples from bench leachate tests. This photo was taken several days after the pears were removed from water and cracks have begun to dry out and collapse. A Pythium lesion is evident on left pear above the grocery label.
Figure 9. Epidermal cracking symptoms in D'Anjou pears. Symptoms in the upper photo developed after prolonged refrigerated storage of the pears in a sealed plastic bag; these pears were not used for baiting. In the lower photo, cracks developed during incubation in water samples from bench leachate tests. This photo was taken several days after the pears were removed from water and cracks have begun to dry out and collapse. A Pythium lesion is evident on left pear above the grocery label.
 Figure 10. Surface pitting of a D'Anjou pear used to bait water from a bench leachate test. The lesions appear to have originated at lenticles and became sunken and corky over time
Figure 10. Surface pitting of a D'Anjou pear used to bait water from a bench leachate test. The lesions appear to have originated at lenticles and became sunken and corky over time

 Figure 11. Epidermal discoloration in Bartlett pear baits due to physiological factors. Upper photo was taken several days after baits were removed from a flooded soil/root sample from container nursery stock. Edges of the discoloration were marked with widely spaced dotted lines at this time. Lower photo was taken a week after the upper photo. Over this time the extent and overall appearance of discoloration did not change.
Figure 11. Epidermal discoloration in Bartlett pear baits due to physiological factors. Upper photo was taken several days after baits were removed from a flooded soil/root sample from container nursery stock. Edges of the discoloration were marked with widely spaced dotted lines at this time. Lower photo was taken a week after the upper photo. Over this time the extent and overall appearance of discoloration did not change.
 Figure 12. Symptoms of scald on D'Anjou pears in a grocery store. This surface abiotic browning is known to develop under some storage conditions. Don't use pears with scald sympotms, or that come from a batch of pears with these symptoms.
Figure 12. Symptoms of scald on D'Anjou pears in a grocery store. This surface abiotic browning is known to develop under some storage conditions. Don't use pears with scald sympotms, or that come from a batch of pears with these symptoms.
Confirmation of Phytophthora infection in baits
Phytophthora infections in pear baits can be confirmed though several methods. Some diagnostic labs will accept pear baits, but check with the lab in advance to determine when they are willing to accept samples. Infected pear baits are highly perishable, and if the lab cannot process them in a timely way, they may become unusable. Pears should be sent or delivered to the processing lab as soon as symptoms are visible. Make sure each pear is labeled. Wrap each pear individually in several layers of clean paper towels and seal each in a labeled zip-closure plastic bag. Carefully pack all bagged baits in a box with enough padding to prevent shifting and bruising. Use next-day delivery if possible; two-day delivery may be acceptable if pears have only early symptoms when shipped and weather is cool.
Direct observation: Pieces of lesions floated in water in petri plates will commonly form sporangia, which can be identified as Phytophthora based on the method of zoospore release. This requires a microscope and training in how to recognize and differentiate Phytophthora from similar microorganisms.
Isolation on media: To isolate Phytophthora from baits onto media, the outer surface of the bait should be disinfested by submerging the pear in in 0.5% NaOCl (diluted bleach) for 30 to 60 seconds. Place the pear on a clean paper towel and let the bleach solution dry off. Alternatively, you can rinse the bleach residue off with a stream of sterile water.
Cut out small pieces from the edges of suspect lesions and place them into agar media in petri dishes. Use aseptic technique throughout and sterilize implements and keep the media from becoming contaminated. Be sure to flame your tools before starting and after each piece is transferred to the media. Pieces should be small, no more than about 3 mm square. Slip the pieces into the agar below the surface. Normally, Phytophthora lesions on pear baits are free of other contaminants so isolations can be made onto general purpose media such as cornmeal agar (CMA), although semi-selective media such as PARP agar may also be used.
Incubate the isolations at root temperature (about 18-24 C [65-75 F]). Check the isolations daily. In general, Phytophthora species grow relatively slowly, increasing in diameter by up to about 1 cm per day. If faster growing colonies appear on the plate (most commonly Pythium species), use sterile technique to cut small pieces from the edges of any potential Phytophthora colonies and transfer them to new petri plates with agar before they are overrun, which will make it impossible to confirm that Phytophthora is present. Mycelium growing out of the tissue pieces should be examined periodically with a microscope. Phytophthora species usually have a characteristic appearance that helps to distinguish them in culture from other genera of water molds and true fungi. A few species can be identified with a high degree of certainty due to their characteristic appearance in culture, but genetic sequencing is generally required to determine the identity of Phytophthora species.
Negative results
It must be understood that baiting, like every other test for the presence of Phytophthora, is subject to false negative results. Negative results may result for a variety of reasons, so a negative result in a single test is not conclusive. Repeated samples from a given infested site can yield both positive and negative results, especially if inoculum levels are moderate to low or infections are distributed nonuniformly. Plants treated with fungicidal materials such as phosphites or mefenoxam may yield negative results but still contain viable (though suppressed) Phytophthora infections. Also, not all Phytophthora species will infect pears, so use of other bait types may be needed. If repeated testing under a range of conditions conducive for Phytophthora detection consistently provides negative results, it is more likely that negative results are meaningful, indicating that Phytophthora is either absent or present in levels that are below the detection threshold. In contrast to negative results, recovery of Phytophthora through baiting is conclusive evidence that the pathogen was present in the sample.
Reference
Erwin, D. C., and Ribeiro, O. K. 1996. Phytophthora Diseases Worldwide. American Phytopathological Society Press, St. Paul, MN.
Updated 12/23/2019 - Revised to reduce overlap and redundancies between this page and 3.2. Detection by baiting - general procedures and details for water samples.







